Use Aktu Btech Quantum Notes to get into VLSI technology. Use these critical, commonly asked questions to master key concepts and dominate your tests. Begin your path to success right away! Unit-4 Diffusion and lon-Implantation
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For VLSI Technology: *Quantum *B.tech-Syllabus *Circulars *B.tech AKTU RESULT * Btech 3rd Year * Aktu Solved Question Paper
Q1. Derive the diffusion equation. How the depth of diffusion is controlled during diffusion process ? Give the solution of Fick’s Law.
Ans. Fick’s law of diffusion :
- 1. Fick assumed that in a dilute liquid or gaseous solution, in the absence of convection, the transfer of solute atoms per unit area in a one dimensional flow can be described by the following equation,



where, J is the rate of transfer of solute per unit area or the diffusion flux,
C is the concentration of solute,
x is the coordinate axis in the direction of the solute flow,
t is the diffusion time, and
D is the diffusivity or diffusion constant.
- 2. Eq.(4.2.1) states that the local rate of transfer (local diffusion rate) of solute per unit area per unit time is proportional to the concentration gradient of the solute, and defines the proportionality constant as the diffusivity of the solute.
- 3. The negative sign on the right-hand side of eq, (4.2.1) states that the matter flows in the direction of decreasing solute concentration (i.e., the gradient is negative).
- 4. Eq. (4.2.1) is called Fick’s first law of diffusion.
- 5. From the law of conservation of matter, the change of solute concentration with time must be the same as the local decrease of the diffusion flux, in the absence of a source or a sink.
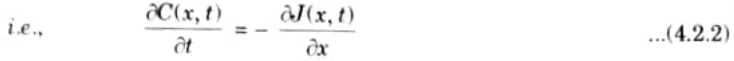
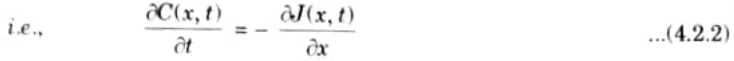
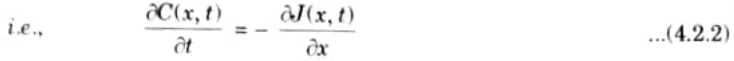
- 6. Substituting eq. (4.2.1) into eq. (4.2.2) yield Fick’s second law of diffusion in one-dimensional form,



- 7. When the concentration of the solute is low, the diffusivity at a given temperature can be considered as a constant, and eq. (4.2.3) becomes,



- 8. Eq. (4.2.4) is often referred to as Fick’s second law of diffusion.
Q2. If the measured phosphorus profile is represented by a Gaussian function with a diffusivity D = 2.3 x 10-13 atoms/cm2, the measured surface dose is 1018 atoms/cm2 and the junction depth is 1 𝛍m at a surface concentration of 1015 atoms/cm3. Calculate the diffusion time.
Ans. Given: D = 2.3x 10-13 atoms/cm2, x= 10-6 m = 10-4 cm, Ns = 1015 atoms/ cm3.
To Find: Diffusion time.
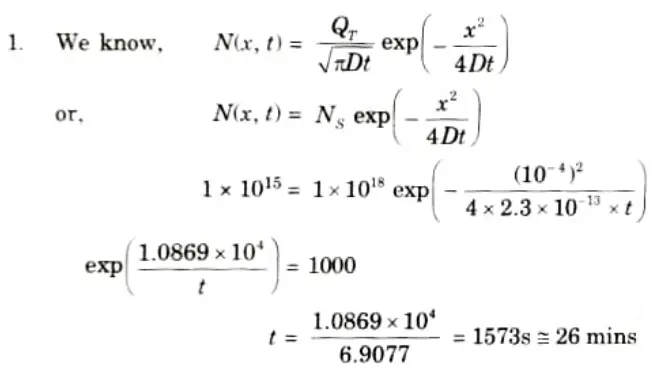
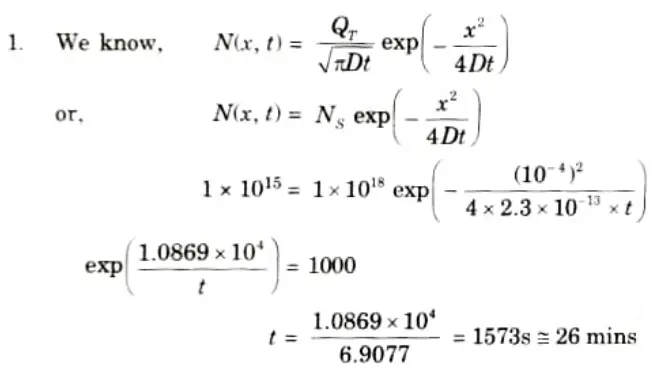
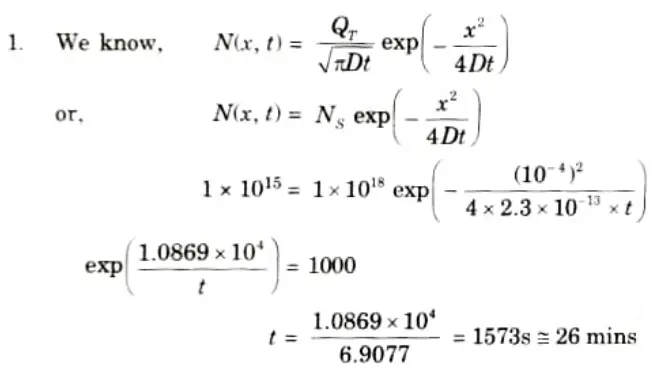
Q3. Describe the impurity behaviour in silicon.
Ans.
- 1. Interstitial diffusion and substitutional diffusion work together to transfer the impurities in groups III and V of the periodic table.
- 2. As a result, the concentration and charge state of lattice point defects have a significant impact on their mobility.
- 3. These consist of the n-type impurities antimony, arsenic, and phosphorus as well as the p-type impurities aluminium, boron, gallium, and indium.
- 4. Groups I and VIll of the periodic table contain the impurities that are capable of moving internally in silicon.
- 5. They include gases like argon, helium, and hydrogen, as well as alkali metals like lithium, potassium, and sodium.
- 6. They are typically electrically inert and occupy interstitial locations in silicon.
- 7. The majority of transition substances diffuse through an interstitial-substitutional mechanism and end up in both kinds of locations.
- 8. In most instances, the mechanism involved is the dissociation of the substitutional into an interstitial and a vacancy.
- 9. The diffusional movement of gold and platinum in silicon is defined by the kick-out mechanism.
Q4. Explain about the deep-ying impurities.
Ans. Deep-lying impurities can be described as follows:
- i.
- 1. These impurities often diffuse five to six orders of magnitude quicker than substitutional diffusers thanks to an interstitial-substitutional process.
- 2. As a result, when the specimens are cooled to room temperature, significant inaccuracy can ensue from out-diffusion effects.
- 3. Rapid quenching attempts typically produce a significant number of crystal flaws and make it difficult to understand the data.
- ii. Their movement is described by a diffusivity which is a function of both concentration and temperature.
- iii.
- 1. The majority of deep-lying contaminants settle in both electronically active and inactive areas after freezing. From one element to the next, the fractions of each type are very different.
- 2. As a result, just 0.1% of nickel is found in active sites, compared to about 90% of gold.
- 3. Because analytical methods like resistivity measurements only give information on the portion that is electronically active, the analysis of the results is complicated.
- 4. Secondary ion mass spectrometry methods, on the other hand, provide data on the complete impurity content.
- iv. The electronically active part of all these dopants exhibits one or more deep levels. Thus their average diffusion parameters cannot be measured by p-n junction techniques, as for substitutional dopants.
- v.
- 1. Clustering seems to be supported by the interaction energy between an interstitial-substitutional diffuser and the strain field connected to a dislocation.
- 2. As a result, it is challenging to appropriately interpret the experimental data since the diffusion process is governed by the crystal’s defect nature.
- 3. A lot of these impurities also combine with silicon at various diffusion temperature ranges to produce compounds.
- 4. They frequently lack electrical activity and cluster together in the silicon lattice.
- 5. Material that has these impurities is typically heat-sensitive.
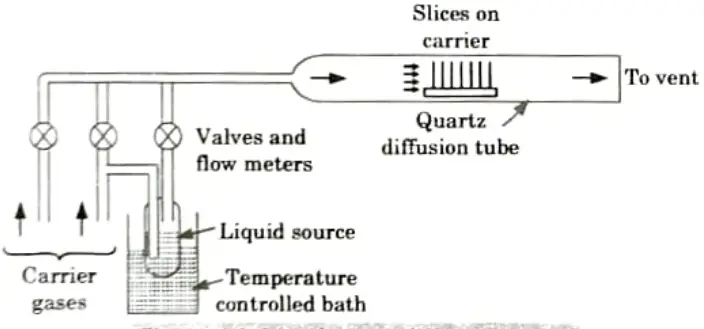
Q5. Discuss gaseous and liquid diffusion systems.
Ans. A. Gaseous source diffusion system:
- 1. Compared to liquid sources, gaseous ones are even more practical.
- 2. Once more, it is customary to employ an excessive amount of dopant gas concentration in order to make these systems less sensitive to the rate of gas flow.
- 3. The concept for a typical diffusion system using a gaseous dopant source is shown in Fig.
- 4. In this case, a carrier gas that is present in the environment and where the diffusion occurs is provided.
- 5. In addition, a chemical trap is frequently included to get rid of harmful unreacted dopant gases.
- 6. All vapour transfer techniques rely on the fact that the surface concentration of the dopant that has been integrated is solid-solubility-limited, making it mostly insensitive to the reactant species’ vapour pressure.
- 7. Still, massive depletion of the source reactant is possible as it travels down the diffusion tube.
- 8. The passage of the reactant vapour between slices results in further depletion.
- 9. Since each slice has a different surface area, it is challenging to maintain doping homogeneity over all of the slices during a diffusion run.
- 10. The current trend towards greater slice sizes and the usage of several slices in a single run, which requires close spacing between individual slices, worsen both of these issues.
B. Liquid-Source diffusion system:
- 1. Liquid-soiree systems are very practical since it is simple to start (or stop) the doping process by controlling the gas through the bubbler.
- 2. In addition, these technologies make it very simple to manage the amount of dopant transferred to the slices by adjusting the bubbler temperature.
- 3. Many halogenic dopant compounds are also offered as liquids.
- 4. By using these sources, heavy metal contamination in diffusion systems is significantly reduced.


Q6. Describe basic layout of implantation equipment.
Ans.
- 1. To send a beam of ions of a specific kind and energy to the surface of a silicon wafer is the fundamental requirement for an ion-implantation system.
- 2. A schematic representation of a medium-energy ion implanter is shown in Fig. As we proceed along the ion route, we start on the left with the high voltage enclosure that houses the majority of the system’s parts.
- 3. A small amount of source gas, such as BP, is fed into the ion source by a gas source, where a heated filament causes the molecules to fracture into charged pieces.
- 4. This ion plasma includes the desired ion as well as numerous additional species from pollution and other pieces.
- 5. An extraction voltage, around 20 kV, causes the charged ions to move out of the ion source into the analyzer.
- 6. The pressure in the remainder of the machine is kept below 10-6 Torr to minimize ion scattering by gas molecules.
- 7. Only ions with the desired charge to mass ratio are allowed to pass through the analyzer’s magnetic field without being stopped by its walls.
- 8. Once the ions transition from a high voltage to the ground, they continue to the acceleration tube where they are accelerated to the implantation energy.
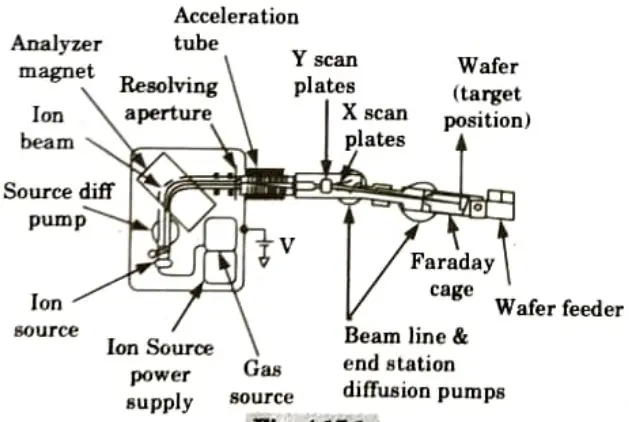


- 9. Apertures guarantee collimation of the beam. Then, using electrostatic deflection plates, the beam is scanned over the wafer’s surface.
- 10. The wafer is somewhat offset from the acceleration tube’s axis to prevent neutralised ions from being deflected onto the wafer.



Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
VLSI Technology Btech Quantum PDF, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Question paper – 2021-22 | 2021-22 |
VLSI Technology Quantum PDF | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One VIew) | Student Result |
