In this Article we are discussing for Important Short Question for Thermodynamics Mechanical Engineering. Hope you Enjoy that session. so Boost Your Knowledge with Short Questions and Answers in Thermodynamics for Mechanical Engineering.
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For Thermodynamics: *Unit-01 *Unit-02 *Unit-03 *Unit-04 *Unit-05 *Short-Q/Ans *Question-Paper with solution 21-22
Unit 01 (Introduction)
Q1. What do you mean by open system ?
Ans. A system is considered to be open if both matter and energy can enter or exit the system.
Q2. What do you mean by isolated system ?
Ans. Systems that exchange neither energy nor matter with other systems or the environment are said to be isolated systems.
Q3. What do you mean by macroscopic approach ?
Ans. The macroscopic approach is a thermodynamic technique that considers a specific amount of matter without taking into account molecular-level occurrences.
Q4. Define microscopic approach.
Ans. The thermodynamic technique known as the microscopic approach assumes that the system is composed of a relatively large number of distinct molecules. These molecules move at various speeds and energy.
Q5. What do you mean by intensive and extensive properties ?
Ans. Intensive Properties : The mass of the system has no bearing on these features. Example : Temperature and pressure.
Extensive Properties : The mass of the system affects these characteristics.
Example: Volume.
Q6. Briefly explain quasi-static process.
Ans. This process is a series of equilibrium states, and its distinguishing quality is infinite slowness. A reversible process is another name for this procedure.
Q7. How does a homogeneous system differ from a heterogeneous system?
Ans. Homogeneous system : A “homogeneous system” is a system that only has one phase. e.g ., milk+ water, aqua ammonia.
Heterogeneous system : A “heterogeneous system” is one that consists of multiple phases. e .g., wet steam, water + mercury.
Q8. What do you mean by non-flow process and flow process ?
Ans. Non-Flow Process : It is the process through which the state of a fixed mass inside the specified boundary changes.
Flow Process : It is the process by which mass flows into and out of an open system’s boundary.
Q9. State steady and unsteady flows.
Ans. Steady Flow : It is a process in which a mass enters the system from the outside and an equal mass exits through the system’s border, keeping the system’s overall mass constant.
Unsteady Flow: It is the process in which the total mass of the system does not remain constant, meaning that the mass at entry and the mass at exit are not equal.
Q10. What do you mean by point function ?
Ans. Two attributes are referred to as a point function when they pinpoint a point on the graph (co-ordinate axes). Examples include volume, temperature, and pressure.
Q11. State zeroth law of thermodynamics.
Ans. According to the zeroth rule of thermodynamics, two systems will be at the same temperature as one another if they are both a third’s temperature apart from one another.
Q12. List any five physical properties of matter which can be used for measurement of temperature.
Ans. 1. Length 2. Radiation
3. Thermal EMF 4. Volume
5. Pressure
Q13. State reversible and irreversible processes.
Ans. Reversible Process : It is described as a procedure that can be stopped at any point and turned around to exactly return the system and environment to their original states. Irreversible Process : It involves the transport of heat through a limited temperature.
Q14. State first law of thermodynamics.
Ans. Heat and work can be converted into one another according to the first law of thermodynamics, but because energy cannot be generated or destroyed, it remains constant throughout an energy conversion.
Q15. Write the limitations of first law of thermodynamics.
Ans. 1. The first law fixes the ratio of heat to work while leaving the direction of change unrestricted.
2. Even if reversing a process does not go against the basic law of thermodynamics, some processes cannot occur except in one direction.
3. The first law of thermodynamics offers a prerequisite for a process to occur, but it is not sufficient in itself.
4. There is a direction law that can be used to determine whether a certain procedure takes place or not. The second law of thermodynamics is based on this.
Q16. What do you mean by work ?
Ans. If a system’s only impact on objects outside of it can be boiled down to the lifting of a weight, then work has been said to have been done.
Q17. Explain free expansion process ?
Ans. Consider a gas that is partitioned off from the vacuum. Allow the divider to be taken out. The volume is quickly filled with gas. Free expansion is the term used to describe a gas expanding against a vacuum.
Q18. Define heat.
Ans. The definition of heat is the type of energy that crosses a boundary as a result of a temperature differential.
Q19. Write Boyle’s law and Charle’s law.
Ans. Boyle’s law: This law states that, while temperature is held constant, the volume of a given mass of a perfect gas varies inversely with absolute pressure.
Product of absolute pressure and volume of a given quantity of gas is constant when the temperature is kept constant.
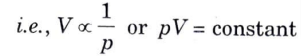
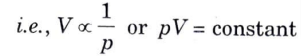
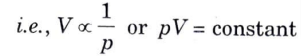
Charle’s Law: According to this law, the volume of a given mass of a perfect gas varies directly with its absolute temperature when pressure is kept constant i.e.,



Q20. What do you mean by throttling process ?
Ans. Throttling process is defined as the expansion of the working fluid across a limitation or extremely small aperture with a steady flow and no heat transfer.
Q21. Define perpetual motion machine of the first kind.
Ans. Since no machine could ever constantly produce mechanical work without causing some other form of energy to vanish at the same time. A first-kind perpetual motion machine is the term used to describe this hypothetical device. Thus, a PMM 1 is not conceivable.
Q22. What do you mean by control surface ?
Ans. The definition of a control surface is the edge of a control volume, a set area in space where focus is placed during problem investigation.
Unit – 2 (Second Law of Thermodynamics)
Q1. Define thermal energy reservoir.
Ans. A huge body with infinite heat capacity is referred to be a thermal energy reservoir if it can absorb or reject an infinite amount of heat without significantly changing its thermodynamic coordinates.
Q2. Define mechanical energy reservoir.
Ans. A big body surrounded by an impermeable adiabatic wall is referred to as a mechanical energy reservoir if it has the capacity to store work as either potential energy or kinetic energy.
Q3. State the various statements of second law of thermodynamics ?
Ans. Kelvin-Planck Statement : According to this, a heat engine cannot produce net work over the course of a whole cycle if it only exchanges heat with objects that have a single constant temperature.
Clausius Statement :It is impossible to build a machine that, when put through a cycle, produces nothing but the transfer of heat from a cooler to a hotter body.
Q4. Define PMM 2.
Ans. The Kelvin-Planck assertion will be broken if heat rejection is zero because the heat engine will only be able to exchange heat with one reservoir for a full cycle before producing net work. A PMM2 is the name of such a heat engine. A PMM2 cannot exist.
Q5. What is energy ?
Ans. The available energy (A.E.) or energy of a cycle heat engine is the greatest work output that can be produced from a given heat input.
Q6. Define heat pump.
Ans. A heat pump is a mechanical device that continuously keeps the body at a temperature higher than the ambient temperature.
Q7. Compare heat pump and refrigerator.
Ans. Compare heat pump and refrigerator :
1. A refrigerator is a thermodynamic device that, during a cycle of operation, rejects heat from a low-temperature body to a high-temperature body in exchange for external work. A heat pump delivers heat energy to a body at higher temperature T1 at the.expense of work energy supplied. A heat pump is generally used to keep the rooms warm in winter.
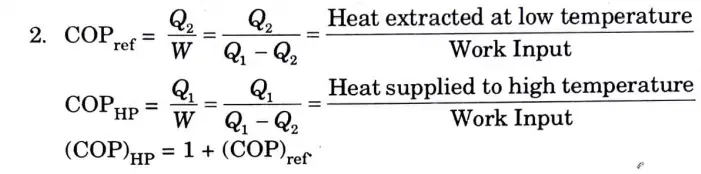
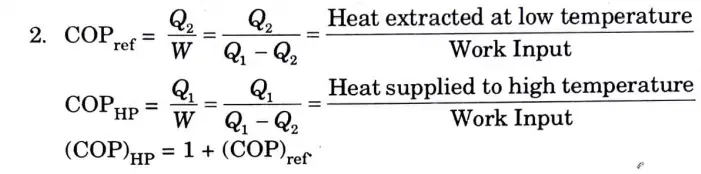
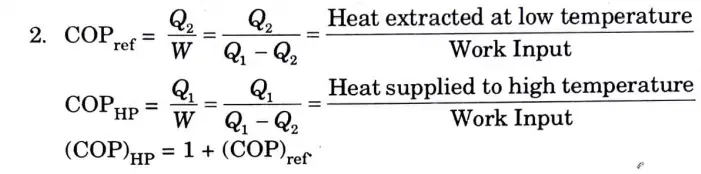
Q8. Define coefficient of performance.
Ans. The ratio of the desired outcome to the work completed is known as the coefficient of performance.
Q9. What are the causes of irreversibility of a process ?
Ans. Following are the main causes of irreversibility of a process:
a. Lack of equilibrium during the process.
b. Involvement of dissipative effects.
Q10. What is the condition for reversibility ?
Ans. When a process is carried out without any kind of dissipative effect and in a way that keeps the system constantly infinitesimally close to a state of thermodynamic equilibrium, it is said to be reversible.
Q11. What do you mean by Carnot cycle ?
Ans. In an ideal hypothetical cycle known as a Carnot cycle, every process that makes up the cycle is reversible.
Q12. State Carnot theorem.
Ans. According to the Carnot theorem, a reversible engine has the highest efficiency of any heat engine that operates between a given constant temperature source and sink.
Q13. What is the corollary of Carnot’s theorem ?
Ans. The efficiency of a reversible engine is independent of the type or quantity of the working substance performing the cycle, according to Carnot’s theorem’s corollary.
Q14. What is the difference between internal and external irreversibility?
Ans. Internal dissipative effects within the system, such as friction, turbulence, etc., are what lead to internal irreversibility. The term “external irreversibility” describes an irreversibility that happens at the system border, such as heat interaction with the environment brought on by a limited temperature gradient.
Q15. Define entropy.
Ans. Entropy is a function of heat quantity that depicts the likelihood of that heat being converted into work.
Q16. What do you mean by Clausius theorem ?
Ans. Clausius theorem states that the cyclic integral of
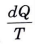
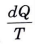
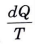
for a T reversible cycle is equal to zero.
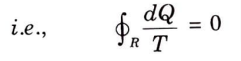
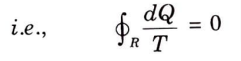
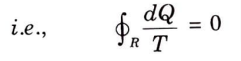
Q17. What is the inequality of Clausius ?
Ans. Inequality of Clausius : According to this,



Q18. What do you mean by entropy principle ?
Ans. This rule states that an isolated system’s entropy can never decrease. It always rises and only stays the same when the process can be reversed.
Mathematically,
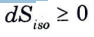
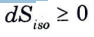
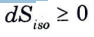
Q19. How will you increase the entropy of a closed system ?
Ans. The entropy of any closed system can increase in two ways :
a. By heat interaction and
b. either through internal irreversibilities or dissipative effects.
Q20. Define entropy generation.
Ans. Entropy or entropy creation refers to the increase in entropy brought on by the production of internal irreversibility. It is denoted by Sgen.
Q21. State third law of thermodynamics.
Ans. The third law of thermodynamics asserts that the maximum degree of order, or zero entropy, is found in a flawless crystal at absolute zero temperature.
Unit – 3 (Availability and Irreversibility)
Q1. What do you mean by available energy and unavailable energy?
Ans. The available energy (A.E.) or exergy of a cycle heat engine is the greatest amount of work output that can be produced from a given heat input. Unavailable energy, also known as anergy, is the absolute minimum amount of energy that must be rejected to the sink in accordance with the second law.
Q2. What is the difference between first and second law of thermodynamics ?
Ans. While the second law emphasises that energy always declines in quality, the first law asserts that energy is always preserved in terms of quantity.
Q3. Define dead state.
Ans. A system must be in a “dead state,” which is defined as a state with zero velocity and the lowest possible potential energy.
Q4. Define availability.
Ans. The amount of meaningful work that can be accomplished in a process by which a system achieves equilibrium with its surroundings is defined as the system’s availability.
Q5. Is the availability function same for a non-flow and a flow process? Justify.
Ans. Availability function is same for a non-flow and a flow process because for both process, the irreversibility is equal to T0∆Sgen . It is equal to decrease inAE or increase in UE.
Q6. Explain reversible process with an example.
Ans. 1. When the initial states of the system and surroundings are restored upon the completion of the reversed process, the process is said to be reversible. This means that neither the system nor its surroundings underwent any unusual modifications.
2. Reversible processes are quasi-static, or processes that go through a series of equilibrium states while moving infinitesimally slowly and with a tiny gradient.
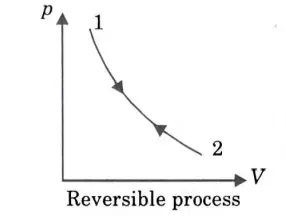


Q7. State the Gouy-Stodola theorem.
Ans. According to the Gouy-Stodola theorem, a process’s rate of accessible energy or exergy loss is proportionate to its rate of entropy production, or vice versa,
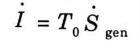
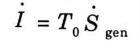
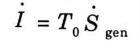
Q8. Define second law efficiency.
Ans. The ratio between the required minimum quantity of accessible energy (also known as exergy) and the actual amount of exergy used to complete a task is known as second law efficiency.
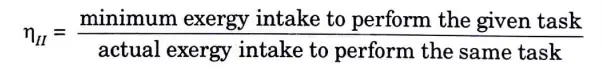
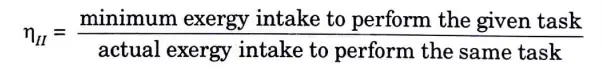
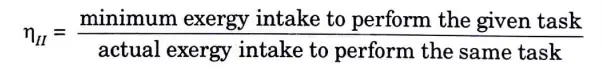
Q9. Define first law efficiency.
Ans. The ratio of a device’s output energy to its input energy is known as first law efficiency.
Q10. What do you mean by Helmholtz function ?
Ans. The term (u – Ts) is known as Helmholtz function, this gives maximum possible output when the heat Q is transferred at constant temperature.
Q11. What are the Maxwell relations ?
Ans. Maxwell Relations:



Q12. What is the importance of Maxwell equations ?
Ans. In thermodynamics, the Maxwell equations are highly helpful because they offer a way to calculate the change in entropy that cannot be detected directly.
Q13. What do you mean by Clausius-Clapeyron equation ?
Ans. Clausius-Clapeyron Equation : The saturation pressure, temperature, enthalpy of evaporation, and the precise volume of the two phases are all related by this equation. In the p-T diagram, it provides the slope of a curve that divides the two phases.
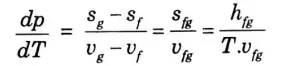
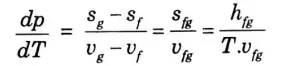
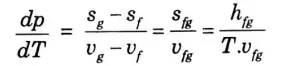
This equation is known as Clausius-Clapeyron equation.
Q14. What is Joule-Thomson coefficient?
Ans. Joule-Thomson Coefficient: The temperature and pressure behaviour of fluids during a throttling process is described by the Joule-Thomson coefficient (µ).
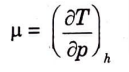
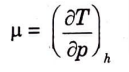
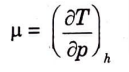
Q15. What is coefficient of volume expansion ?
Ans. Coefficient of Volume Expansion: It is defined as,
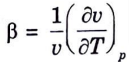
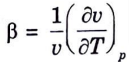
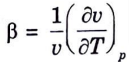
This also goes by the name “volume expansivity.” It measures the volume change with temperature while maintaining a constant pressure.
Q16. What do you mean by adiabatic compressibility?
Ans. Adiabatic Compressibility: It is represented by following relation,
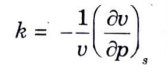
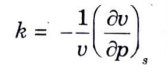
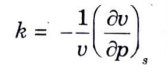
Unit – 4 (Properties of Steam and Rankine Cycle)
Q1. Define degree of superheat.
Ans. The term “superheat” or “degree of superheat” refers to the temperature difference between the superheated vapour and the saturation temperature at that pressure.
Q2. Define triple point.
Ans. The term “triple point” refers to the temperature and pressure conditions at which a substance can coexist in equilibrium in all three of its states (solid, liquid, and vapour).
Q3. State critical point.
Ans. The critical point is where there is no longer a difference between the entropies of water and dry steam.
Q4. Define dryness fraction of steam ?
Ans. The mass of dry steam present in a known amount of wet steam divided by the total mass of steam is known as the dryness fraction of steam.
Mathematically,
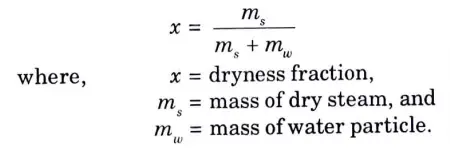
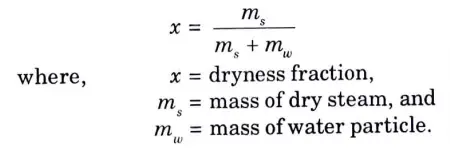
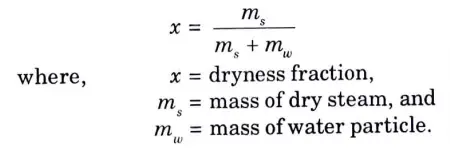
Q5. What do you mean by external work and internal latent heat?
Ans. External Work: There is a large increase in volume during the transition of the liquid into the vapour state when water evaporates under specific conditions of pressure and temperature. Steam performs the function by raising its volume in this manner. This technique is referred to as external evaporation work.
Internal Latent Heat: In order to expand the volume and create external work, a particular amount of latent heat added to water during the evaporation process is used.
Internal latent heat is the difference between the latent heat of steam and the exterior work of evaporation.
Q6. What is a saturation state ?
Ans. A phase change may take place without a change in pressure or temperature in the saturation state.
Q7. What do you mean by vapour dome?
Ans. The saturated or vapour dome is formed when the saturated liquid line with regard to vapourization and the saturated vapour line slant toward one another.
Q8. How does triple point exist on the P-V diagram ?
Ans. On the P-V diagram, the triple point is a location where the equilibrium between all three phases—solid, liquid, and gas—is achieved.
Q9. What advantages are obtained if superheated steam is used in steam prime movers ?
Ans. It boosts the productivity of prime movers, which immediately boosts effectiveness.
Q10. What are the different types of calorimeters ?
Ans. Following are the types of calorimeters :
1. Separating calorimeter,
2. Throttling calorimeter,
3. Combined separating and throttling calorimeter, and
4. Barrel calorimeter.
Q11. Define psychrometry.
Ans. According to its definition, psychrometry is the area of engineering science that deals with the study of wet air, or dry air that has been mixed with humidity or water vapour.
Q12. What do you mean by specific humidity ?
Ans. SpIn a mixture of dry air and water vapour, specific humidity is the weight of water vapour per kilogramme of dry air. Both humidity ratio and humidity are other names for it. And it is denoted by ω.
It is expressed in terms of kg per kg of dry air.
Q13. What is absolute humidity ?
Ans. Absolute humidity is defined as the amount of water vapour present in kg in 1 m3 volume of mixture of dry air and water vapour.
Q14. What do you mean by relative humidity?
Ans. The ratio of the actual water vapour content in a given volume of a mixture to the greatest quantity of water vapour that might be present in that same volume at the same temperature when the mixture is saturated is known as relative humidity.
It is denoted by RH or Φ.
Q15. What is dry bulb temperature ?
Ans. The temperature measured by a standard thermometer that is unaffected by thermal radiation or airborne moisture is known as the dry bulb temperature (DBT).
Q16. Define wet bulb temperature.
Ans. The temperature read by a thermometer whose bulb is covered in a wet cloth and exposed to moving air is known as the wet bulb temperature (atmospheric air).
Q17. What is wet bulb depression ?
Ans. Dry bulb temperature minus wet bulb temperature equals wet bulb depression.
WBD = DBT- WBT
Q18. What do you mean by dew point temperature (DPT) ?
Ans. When the mixture of moist air is cooled under constant pressure, the water vapour present just starts to condense. This temperature is known as the dew point temperature (DPT).
Q19. What is dew point depression ?
Ans. The difference between the dry bulb temperature and the air’s dew point is known as the dew point depression.
DPD = DBT – DPT
Q20. Define degree of saturation.
Ans. The ratio between the actual humidity level and the saturated humidity level, both at the same temperature and total barometric pressure, is known as the degree of saturation.
It is denoted byµ.
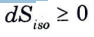
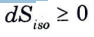
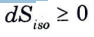
where, ω = actual humidity ratio, and
ωs = saturated humidity ratio.
Q21. Define bypass factor.
Ans. The ratio of the actual temperature change to the maximum temperature change is known as the bypass factor. It is denoted by x. For heating,



Q22. Define humidification and dehumidification.
Ans. Humidification : Humidification is the process of adding moisture to the air without affecting the dry bulb temperature.
Dehumidification : Dehumidification is the process of removing moisture from the air without affecting the dry bulb temperature.
Q23. What do you mean by sensible heat factor ?
Ans. Since sensible heat and latent heat can both be added during psychrometric processes. Sensible heat factor (SHF) or sensible heat ratio are terms for the proportion of sensible heat to total heat (SHR).
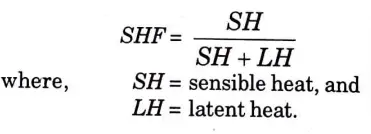
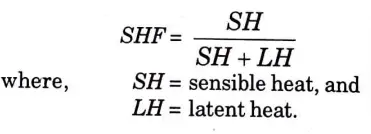
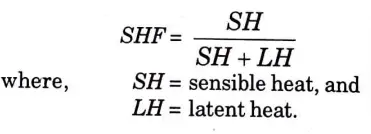
Q24. What do you understand by adiabatic mixing of two air streams?
Ans. The final state of an air mixture created by mixing two volumes of air with various enthalpies and specific humidity relies on the masses involved as well as the enthalpies and specific humidity of each of the constituent masses that make up the mixture. Adiabatic mixing is the term used to describe this kind of air stream mixing.
Unit – 5 (Refrigerator Cycles)
Q1. What do you mean by refrigeration ?
Ans. Refrigeration: It is the science of creating and maintaining temperatures lower than the atmosphere around an object, or eliminating heat from it under controlled circumstances.
Q2. Write two application of refrigeration.
Ans. Application of Refrigeration :
1. Making of ice, and
2. Used in transportation of food at a required temperature.
Q3. What is unit of refrigeration ?
Ans. Tonnes of refrigeration serve as the practical refrigeration unit. TR is how it is written.
Q4. Define a tonne of refrigeration.
Ans. Tonne of Refrigeration : It is determined by the uniform melting of one tonne (1000 kg) of ice over the course of a 24-hour period at 0°C.
Practically,
1 TR = 210 kJ / min
Q5. Define refrigeration effect.
Ans. Refrigeration Effect : The refrigeration effect is the quantity of heat removed from the system.
Q6. Define coefficient of performance of a refrigerator.
Ans. Coefficient of Performance of a Refrigerator: The ratio of heat removed from the refrigerator to the work done on the refrigerant is what is meant by this term.
Mathematically,



Q7. Define refrigerator.
Ans. Refrigerator: It is a type of reversed heat engine that lowers or maintains a body’s temperature below that of the surrounding air.



Q8. Name the systems by which mechanical refrigeration can be accomplished.
Ans. Mechanical refrigeration can be accomplished with :
- 1. Compressor,
- 2. Evaporator,
- 3. Expander, and
- 4. Condenser.
Q9. What are the components of Carnot refrigeration cycle ?
Ans. Carnot Refrigeration Cycle : It consists of four basic components :
- 1. Compressor,
- 2. A heat rejection device,
- 3. An expander coupled to the compressor, and
- 4. A cold chamber.
Q10. Write two merit and demerit of air refrigeration system.
Ans. Merits:
1. Both air and refrigerant are cheap and readily available.
2. The air is non-toxic and non-inflammable.
Demerits:
1. Low COP.
2. There is a sizable rate of air circulation.
Q11. Write the components of a Bell-Coleman cycle.
Ans. A Bell-Coleman cycle consists of the following components :
1. Compressor,
2. Cooler,
3. Expander, and
4. Refrigerator.
Q12. Write two advantage and two disadvantage of VCR system over air refrigeration system ?
Ans. Advantages :
1. It is smaller than expected considering the refrigeration capacity.
2. The coefficient of performance is quite high .
Disadvantages :
1. The initial cost is high.
2. The main issue with a vapour compression system is preventing refrigerant leaks.
Q13. What are the types of vapour compression cycles ?
Ans. Following are the types of vapour compression cycles :
- 1. After compression, cycle with dry saturated vapour.
- 2. Following compression, perform a wet vapour cycle.
- 3. After compression, do a superheated vapour cycle.
- 4. Before compression, cycle with superheated vapour.
- 5. Cycle with refrigerant undercooling or subcooling.
Q14. How will you improve simple saturation cycle?
Ans. The simple saturation cycle may be improved by the following methods:
- 1. By positioning the flash chamber between the evaporator and the expansion valve.
- 2. By employing the pre-cooler or accumulator.
- 3. By vapour refrigerant subcooling the liquid refrigerant
- 4. By subcooling the liquid refrigerant leaving the condenser by liquid refrigerant from the expansion valve.
Q15. What will be the effect of superheating the vapour before suction to compression in a VCR system ?
Ans. Effects of Superheating:
1. Makes compression work harder.
2. Improves the condenser’s ability to reject heat.
3. Can raise or lower COP.
Q16. What will be the effect of suction pressure on decreasing its value in a VCR system?
Ans. For the same quantity of refrigerant flow, the COP of the cooling system will be reduced. As a result, the system’s cooling capacity would drop and refrigeration costs will rise.
Q17. Define refrigerant?
Ans. In the course of its cycle in the refrigeration system, refrigerant is a heat-conveying medium that transfers heat from a low-temperature system to a higher-temperature system.
Q18. Define primary and secondary refrigerant.
Ans. Primary Refrigerant: Primary refrigerants are those which immediately contribute to the refrigeration system. For example : R-12, NH3 and SO2 etc.
Secondary Refrigerant: Secondary refrigerants are those that are utilized for cooling after being first cooled by primary refrigerants.
For example : H2O, brine solution of NaCl and CaCl2 etc.
Q19. Define azeotrope refrigerants with examples.
Ans. Azeotrope Refrigerants : These are referred to as a stable blend of refrigerants whose liquid and vapour phases maintain the same chemical compositions over a broad temperature range. Examples:
1. R-500, and
2. R-502.
Q20. Give two examples of inorganic refrigerants.
Ans. Following are the two examples of inorganic refrigerants :
1. R-717 (ammonia), and
2. R-729 (air).
Q21. Write down desirable properties of refrigerants.
Ans. 1. Low boiling point
2. Low specific heat of liquid
3. Low specific volume of vapour
4. Low cost
5. Non toxic and non corrosive to metal.
Q22. What is the function of absorber in VAR system ?
Ans. In order to form a strong or rich solution of the refrigerant in the absorbent or adsorbent, the absorber is employed to absorb the refrigerant vapour by its weak or poor solution in a suitable absorbent or adsorbent.



Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
Thermodynamics Quantum, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Important Unit-1 | Unit-1 |
| Important Unit-2 | Unit-2 |
| Important Unit-3 | Unit-3 |
| Important Unit-4 | Unit-4 |
| Important Unit-5 | Unit-5 |
| Question paper – 2021-22 | 2021-22 |
Thermodynamics Quantum PDF: | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One VIew) | Student Result |

3 thoughts on “Short Questions and Answers in Thermodynamics for Mechanical Engineering”