Here we are discussing for Unit 01 introduction – Thermodynamics Mechanical Engineering
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For Thermodynamics: *Unit-01 *Unit-02 *Unit-03 *Unit-04 *Unit-05 *Short-Q/Ans *Question-Paper with solution 21-22
Q1. Write a short note on following:
a. System,
b. Boundary, and
c. Surroundings.
d. Macroscopic and Microscopic Approaches
Ans. a. System : A system is a limited amount of stuff or a designated area of space that is the subject of a thermodynamic research.
i. Closed System :A closed system is one in which the system boundary is impenetrable to the mass flow. No mass may enter or leave the system. Energy can pass the border in both the forms of heat and work.
ii. Open System : A system that permits both mass and energy interactions with the environment is said to be open.
iii. Isolated System : Any system that doesn’t exchange mass or energy with its surroundings is said to be isolated.
b. Boundary:
1. The actual or hypothetical envelope enclosing a system is the boundary of the system.
2. The boundary may be real or hypothetical (i.e., imaginary, Fig.)
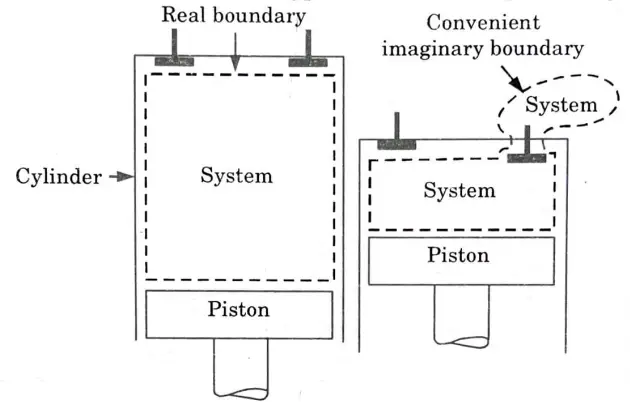


3. It may be rigid or it may move (e.g., as and when a system containing a gas is compressed or expanded).
c. Surroundings : Anything outside the system boundary is termed as surroundings.
Universe= Systems+ Surroundings
d. Macroscopic and Microscopic Approaches :
1. Atomic or molecular research on thermodynamics is possible. Consider the creation of a helium nucleus by the fusion of four hydrogen nuclei (protons), for instance. This method is tiny.
2. In contrast, a macroscopic method refers to a thermodynamic analysis of a sizable collection of atoms or molecules.
Q2. Describe thermodynamic equilibrium of a system.
Ans. A. Thermodynamic Equilibrium :
1. If a system is isolated from its environment, it is said to be in a condition of thermodynamic equilibrium when no spontaneous change in any macroscopic property is observed. There cannot be a spontaneous change in any macroscopic property of the system if it is in equilibrium.
2. If the following three categories of equilibrium are met, a system will be in thermodynamic equilibrium.
a. Mechanical Equilibrium:
1. If there are no pressure forces that are out of balance both within the system and between the system and its surroundings, the system is in mechanical equilibrium.
b. Chemical Equilibrium:
1. If there is no chemical reaction, or transfer of matter from one part of system to another, (such as diffusion or solution), system is in chemical equilibrium.
c. Thermal Equilibrium:
1. Condition or state in which the temperature of system is uniform.
Q3. Define Dalton’s law and Amagat’s law.
Ans. A. Dalton’s Law of Partial Pressure :
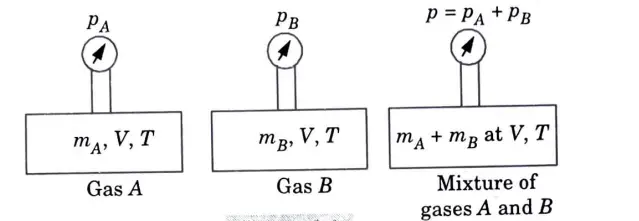
1. This rule states that “the total pressure exerted by a mixture of gases equals the algebraic sum of the partial pressures exerted by each ingredient when they occupy the same volume and temperature of the mixture.”
2. Consider two individual gases A and B which occupy volume Vat temperature T are mixed together and kept in a third container of volume V and temperature T.
Volume of gas A= Volume of gas B
= Volume of mixture (A + B)


From mass balance equation,
m = mA + mB
From Dalton’s law of partial pressure,
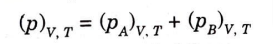
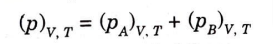
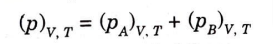
Above equations can be extended for any number of non-reactive gases.
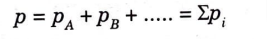
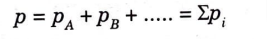
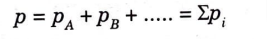
B. Gibbs Dalton’s Law :
1. This law states that “when individual gases occupy the same volume and temperature of the mixture of gases, the internal energy, enthalpy, and entropy of the mixture of gases is equal to the algebraic sum of internal energies, enthalpies, and entropies of individual gases.”
2. From this law, following relations can be written
3. Although Gibbs Dalton’s law is applicable to perfect gases, it can be applied to real gases for approximate engineering calculations involving mixture of gases at lower pressure.
C. Amagat’s Law :
1. This law states that “the total volume filled by a mixture of gases equals the sum of the volumes which would be occupied by each ingredient, when they are at the same pressure and temperature as that of the mixture.”
2. It follows that
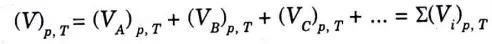
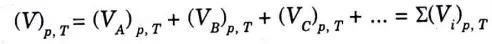
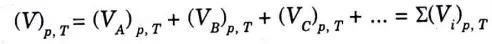
Q4. Write down zeroth law of thermodynamics. Explain how it can be used for temperature measurement.
Ans. A. Zeroth Law of Thermodynamics :
1. B and C will be in thermal equilibrium with one another if a body A is in thermal equilibrium with both a body B and, independently, a body C. The zeroth law of thermodynamics is referred to as this.
B. Temperature Measurement :
1. Temperature measurement is based on the zeroth law. By placing a body in touch with another one (like a thermometer) and allowing thermal equilibrium to occur, one can ascertain the temperature of the first body.
2. A thermometer’s temperature-dependent characteristic is measured to determine the temperature value. The term “thermometric property” refers to this kind of property of thermometer, which can include things like gas volume, gas pressure, solid electrical resistance, magnetic effects, etc.
3. It is essential to create a temperature scale on which the temperature of a body or system may be read in order to quantify the thermal state of a body.
4. It needs selection of basic unit and a reference state. For this purpose, generally two fixed points are used :
i. Ice point: Ice point is the temperature at which ice and air-saturated water are in equilibrium at standard atmospheric pressure.
ii. Steam point: At standard atmospheric pressure, this is the temperature at which pure water and its own vapour are in equilibrium.
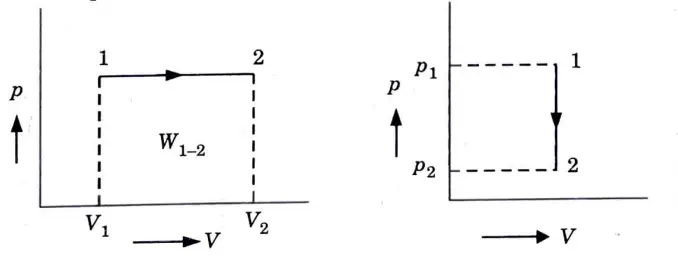
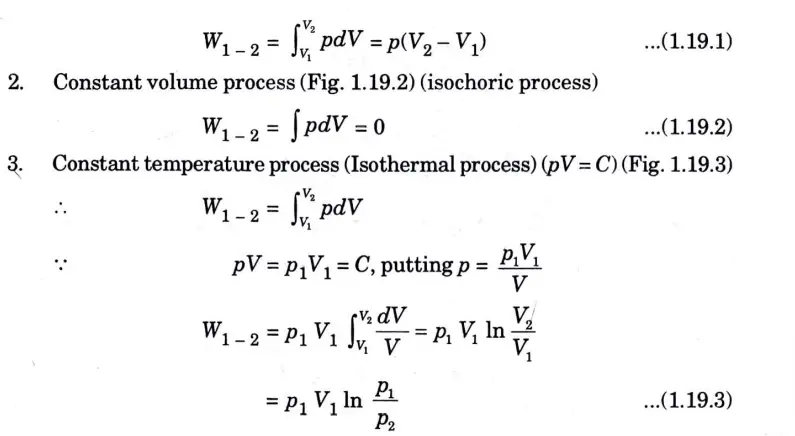
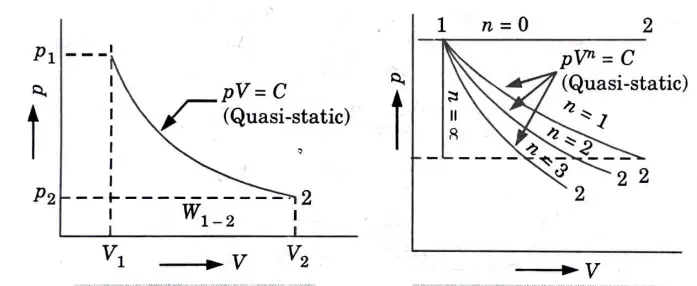
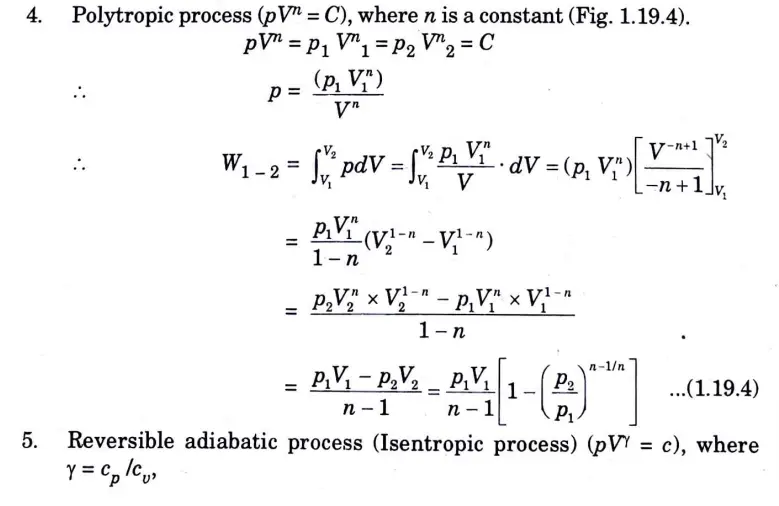
Q5. Derive the expression of work done for various non-flow processes .
Ans. 1. Constant pressure process (Fig. 1) (isobaric or isopiestic process)








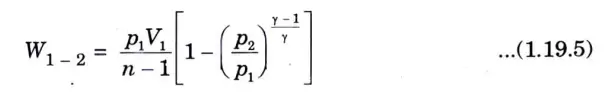
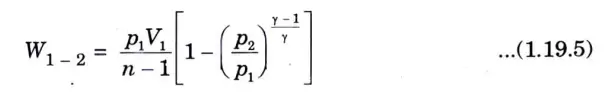
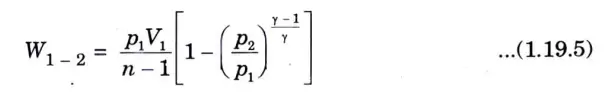
Q6. What are the applications of SFEE?
Ans. A. Application of SFEE to Engineering Devices :
a. Nozzle and Diffuser:
1. The flow through a nozzle is characterized by following features :
i. Shaft work is zero i.e., Ws = 0
ii. If the flow is reversible adiabatic manner, then Q = 0.
iii. If the nozzle is-horizontal, change in elevation, i.e., dZ will be zero
∴ Z1 = Z2
2. Under these features, SFEE for a nozzle / diffuser is reduced to
3. For a nozzle
Enthalpy drop = Increase in kinetic energy
4. For a diffuser,
Rise in enthalpy = decrease in kinetic energy.
b. Boiler:
1. A boiler has following features :
i. Shaft work is zero, Ws = 0.
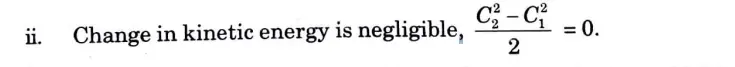
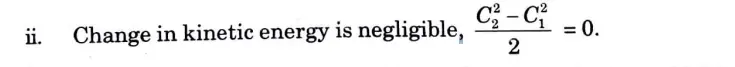
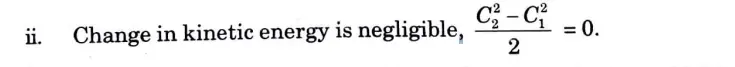
iii. Change in elevation between inlet and outlet point is negligible,
Z1 =Z2
2. Therefore SFEE is reduced to
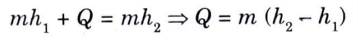
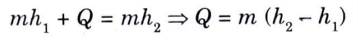
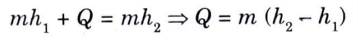
c. Turbine:
1. A steam or gas turbine has following features :
i. ΔKE or d (kinetic energy)= 0
ii. ΔPE or d (potential energy)= 0
iii. Q = 0 since walls are insulated.
2. Therefore, SFEE for a turbine is reduced to
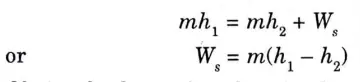
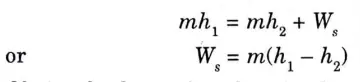
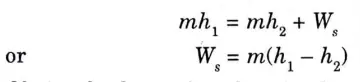
Obviously the work is done by the turbine at the expense of enthalpy.
d. Compressor :
1. A compressor is characterized by following features :
i. Shaft work is negative
i.e., Ws = negative
(Since work is done on the system and working fluid is compressed.)
ii. Change in potential energy is negligible
i.e ., d(PE) = 0
iii. Generally heat is lost to surroundings, Q is negative.
2. Therefore SFEE for a compressor is reduced to
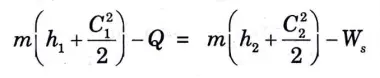
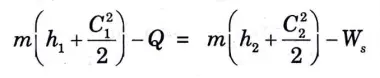
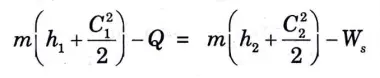
e. Centrifugal Water Pump:
1. For a pump, Q = 0, dU = 0
(Since there is no change in the temperature of water)
2. Work is negative since it is done on the system.
3. SFEE for a pump is reduced to
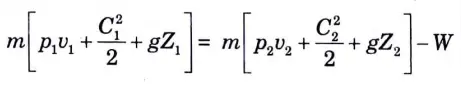
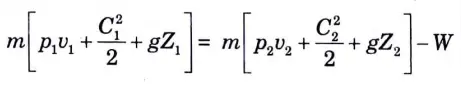
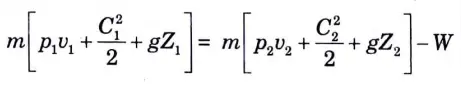
f. Heat Exchanger :
1. A heat exchanger is characterized by the following features :
i Shaft work is zero, Ws = 0
ii. Change in KE = 0
iii. Change in PE = 0
iv. It is a perfectly insulated system i.e., no external heat interaction.
2. From energy balance equation, we can write ,
Energy given by fluid A = Energy gained by fluid B



Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
Thermodynamics Quantum, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Important Unit-1 | Unit-1 |
| Important Unit-2 | Unit-2 |
| Important Unit-3 | Unit-3 |
| Important Unit-4 | Unit-4 |
| Important Unit-5 | Unit-5 |
| Question paper – 2021-22 | 2021-22 |
Thermodynamics Quantum PDF: | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One VIew) | Student Result |

6 thoughts on “Unit 01 Introduction – Thermodynamics Mechanical Engineering”