Availability and irreversibility are essential concepts in thermodynamics that play a significant role in defining the efficiency and effectiveness of energy conversion systems. This course delves into the essential ideas of Availability and Irreversibility, as well as provides critical concerns for students to address.
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For Thermodynamics: *Unit-01 *Unit-02 *Unit-03 *Unit-04 *Unit-05 *Short-Q/Ans *Question-Paper with solution 21-22
Ans. A. Available and Unavailable Energy:
- 1. Available energy is the portion of the heat energy supplied into a cyclic heat engine that is transformed into mechanical work.
- 2. “Unavailable energy” refers to that portion of heat energy that cannot be used and must be rejected to the environment.
- 3. The term ‘exergy’ is synonymous with available energy and the term ‘anergy’ is synonymous with unavailable energy. Therefore, Energy= exergy + anergy.
- 4. The concept of availability refers to the greatest theoretical work that can be extracted from a system at a given state up until it is in a dead state (without dissipative effects).
- 5. The maximum useful work produced under such ideal circumstances is referred to as the system’s available energy, while the portion of energy rejected is referred to as the system’s unavailable energy.
- 6. It is important to keep in mind that, even though the system still has internal energy, it is not possible to refer to this energy as available energy when the system achieves its dead state.
Q2. Explain the availability of a closed system.
Ans. A. Availability of a Closed System:
1. Let’s consider a piston cylinder arrangement substance at p1,T1 and V1 is expanded reversibly to close state at p0, V0 and T0.
2. The working substance expands and Wwxpansion is obtained.
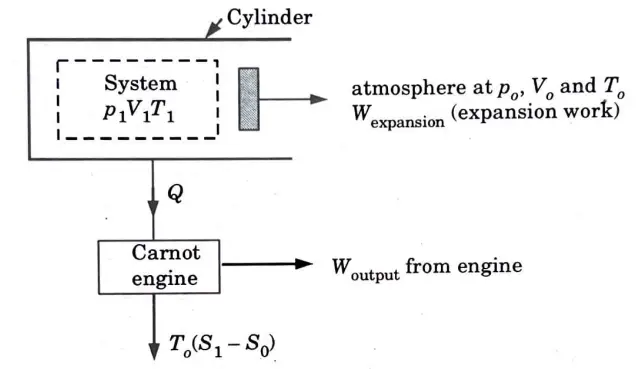


3. From the law of conservation of energy,
Q = dU+W
– Q = Wexpansion + (U0 – U1)
(Heat interaction is negative because it leaves the system.)
Therefore, Wexpansion = (U1 – ,U0) – Q
4. This heat rejected from the piston-cylinder arrangement can be used to run a reversible heat engine. Work output of the reversible engine is equal to
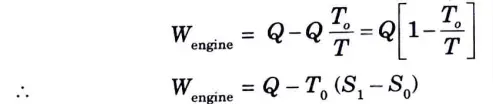


5. Maximum work obtained from the cylinder piston assembly is the sum of Wexpansion and Wengine.
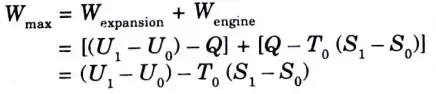
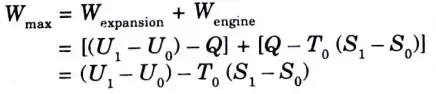
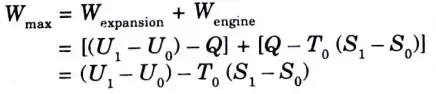
6. Surrounding work, Wsurrounding = p0 (V0 – V1)
This is the work which the piston, while moving outwards, has to spend in pushing the atmosphere against its own pressure.
7. Therefore maximum available useful network,
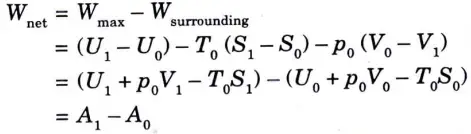
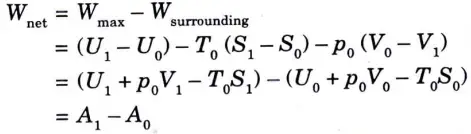
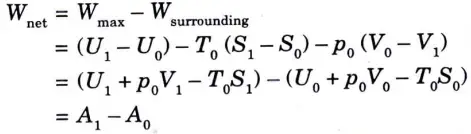
Where A= U + p0 V -T0 S is called non-flow availability function.
8. This is a composite property of the system and surroundings because it consists of extensive properties (internal energy U, volume V and entropy S) and intensive property environment pressure p0 and temperature T0). Thus availability is a function of properties of the surroundings and also of end states of the closed system.
9. Entropy change for a closed system can be calculated from the relation;
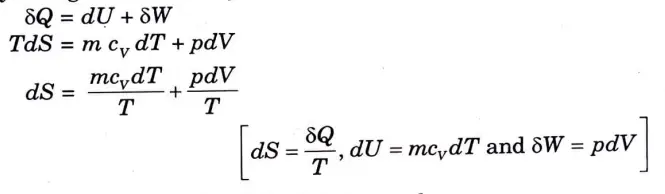
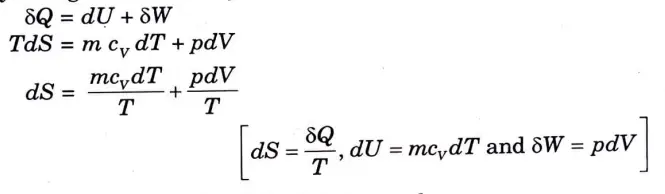
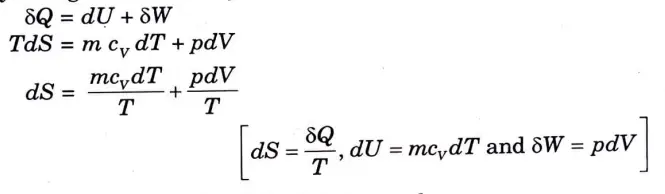
10. Integrating between initial and final states, we have
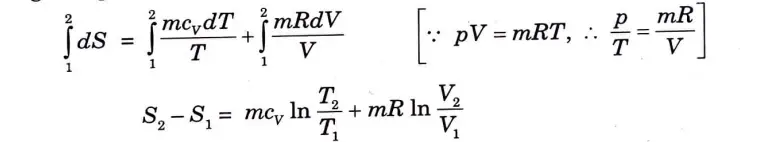
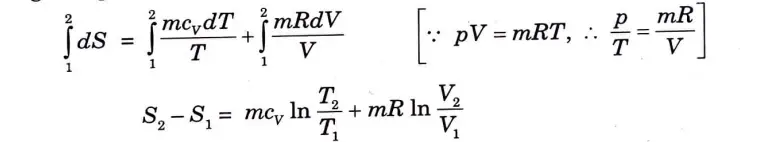
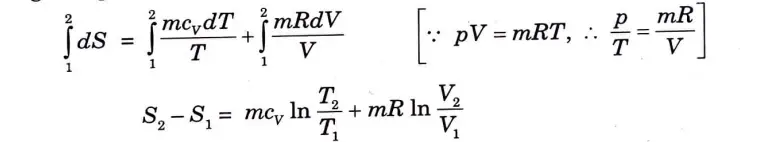
Q3. What is meant by irreversibility and effectiveness of systems?
Ans. 1. The system’s maximum useful work (or network) is a reversible process in which the system interacts with its surroundings by producing heat. Since processes in real-world settings are irreversible, the system’s actual work for a given state change is never greater than reversible work.
2. Therefore, irreversibility of a process is defined as the difference of reversible work and the actual work.
3. In mathematical form,
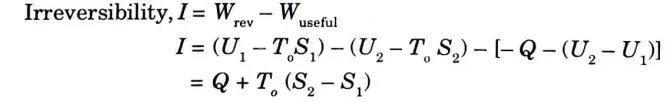
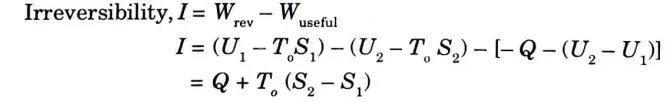
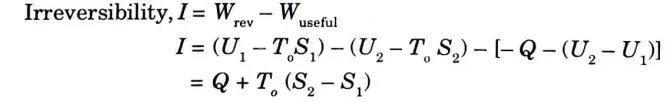
4. Change in entropy of surroundings due to heat addition at constant atmospheric temperature, T0 is



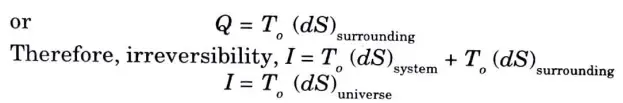
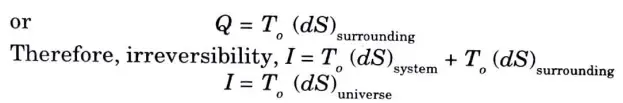
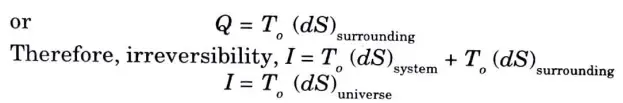
5. From the above expression irreversibility equals the product of entropy produc\ion and surroundings temperature. 6. The expression T0 (dS)universe also represents an increase in the unavailable part of energy (or Anergy) and therefore it can be stated that irreversibility causes an increase in Anergy.
Q4. What is the importance of availability, effectiveness and irreversibility ?
Ans. 1. The first law of thermodynamics gave rise to the idea of efficiency, which is exclusively applicable to cycles, but the second law of thermodynamics gave rise to the concepts of availability, irreversibility, and effectiveness, which are applicable to both processes and cycles.
2. These ideas also imply an improvement in thermodynamic cycles since they highlight the differences between actual processes and ideal processes, which makes it easier to analyse processes.
3. Since the first rule of thermodynamics does not predict any irreversibility during the process, the derived notions of availability, irreversibility, and efficacy are especially helpful in heat transfer procedures involving two fluids.
Q5. Define dead state and second law efficiency.
Ans. A. Dead State:
1. When a system and its surroundings are in a condition of chemical, thermal, and mechanical equilibrium, the term “dead state” is used.
2. As a result, neither a spontaneous change in the environment nor a spontaneous interaction between the system and its surroundings are possible. Restricted dead state refers to a dead state that is a limiting state.
3. The system has no kinetic or potential energy in relation to its surroundings when it is in a dead state, which has the same temperature and pressure as its surrounds.
4. As a result, a system can only produce its greatest amount of work when it follows a reversible process from its current state to that of its surroundings. Otherwise, it will have 0% availability at the dead state (dead state).
B. Second Law Efficiency :
1. The second law efficiency ” ηII “of a process is defined as the ratio of the minimum available energy which must be consumed to do a work divided by the actual amount of available energy consumed in performing the work.



Where, A is the availability or exergy.
Q6. Prove that in a closed system, when initial and final temperatures are equal to that of the environment and the system exchanges heat with the environment only, the work done is either equal to or greater than the change in the Helmholtz function.
Ans. 1. Let’s consider a closed system which is initially and finally at surroundings temperature around it and has heat interactions with the atmospher e only,
2. From first law of thermodynamics,
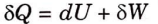
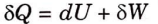
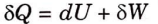
3. From the second law of thermodynamics,
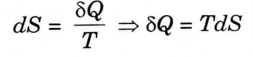
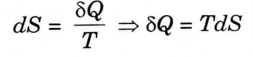
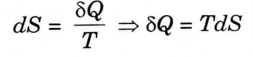
4. For a non-flow reversible process,
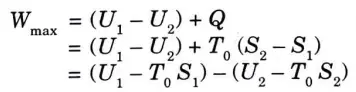
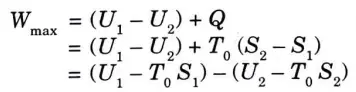
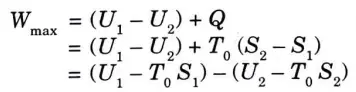
5. Since the process is taking place at constant temperature, T0 can be replaced by T1 or T2.
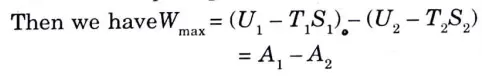
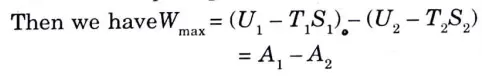
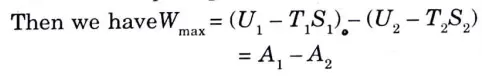
6. The term ( U – TS) is known as Helmholtz function and can be defined as the difference between the internal energy and the product of entropy and temperature. 7. If a closed system passes from one state to another state at same temperature while interacting heat only with the surrounding atmosphere at T0 = T1 = T2, the maximum work obtained from the process is equal to the decrease in Helmholtz function of the system.



Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
Thermodynamics Quantum, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Important Unit-1 | Unit-1 |
| Important Unit-2 | Unit-2 |
| Important Unit-3 | Unit-3 |
| Important Unit-4 | Unit-4 |
| Important Unit-5 | Unit-5 |
| Question paper – 2021-22 | 2021-22 |
Thermodynamics Quantum PDF: | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One VIew) | Student Result |

5 thoughts on “Unit 3: Important Questions on Availability and Irreversibility in Thermodynamics”