Understanding the basics of steam and the Rankine cycle is critical in mechanical engineering. This section delves into the fundamental principles of steam and the Rankine cycle, such as thermodynamics cycles, heat transmission, steam characteristics, and power generation. The Rankine cycle and its components, such as pumps, turbines, and condensers, will be taught to students.
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For Thermodynamics: *Unit-01 *Unit-02 *Unit-03 *Unit-04 *Unit-05 *Short-Q/Ans *Question-Paper with solution 21-22
Q1. What is meant by pure substance ?
Ans. 1. A pure substance is described as a single-component system that, regardless of its phase, has a homogeneous and consistent chemical makeup.
2. A pure substance may exist in one of the following states: solid (as ice), liquid (as water), gaseous (as vapour), or mixed (as ice plus water plus vapour).
3. For carrying out thermodynamic operations, pure substances are the working materials.
4. As a result, a pure substance is one whose chemical composition is homogeneous and stays constant during the transmission of heat and work with the environment, such as water, ice, vapour, oxygen, nitrogen, etc.
5. Heat transmission with the environment may cause its phase proportions to change.
Q2. Draw the p-T diagram of pure substance and explain its various regions of the diagram in details.
Ans. A. p-T Diagram of Pure Substance :
1. Substances can exist in liquid, solid, and vapour forms, respectively (gas). These “phases,” as they are known, are created when the substance absorbs or emits heat.
2. Solid phase changes while heating from solid to liquid to vapour or gaseous phase. Phase transformation from a gas (vapour) to a liquid and then a solid state is reversed upon cooling.
3. The “transition temperature” is the temperature at which the phase shift occurs.
4. For each substance, there is a set of pressure and temperature at which any two out of three phases may co-exist in equilibrium.
5. The Fig: drawn below represents different phases of a substance as a function of pressure and temperature.
6. The Fig. imparts following valuable information :
a. Solid and Liquid Phases :
- 1. The line TA divides the equilibrium coexistence of the solid and liquid phases.
- 2. The “fusion curve,” or line TA, depicts how the melting point varies with pressure and temperature.
- 3. The melting point lowers with increasing pressure, according to the line TA’s leftward slope. These qualities define “ice type compounds.”
b. Liquid and Vapour Phases :
- 1. Liquid and vapour phases co-exist in equilibrium and are separated by the line TB.
- 2. The line TB is known as “vaporization curve” vaporization curve has an upper end point (or upper critical point) above which no substance can exist in liquid state whatever amount of pressure is applied on it. ·
c. Vapour and Solid Phases :
- 1. Vapour and solid phases coexist in equilibrium and are separated by the line TC. The line TC is known as “sublimation curve”.
- 2. Sublimation is defined as the process during which a solid phase is directly changed into a vapour (gas) phase or vice-versa.
d. Triple Point :
- 1. A triple point is the temperature and pressure combination at which a substance can dwell in equilibrium in all three of its states (solid, liquid, and vapour).
- 2. Triple point is shown by the point T in the Fig. At triple point, all the three curves namely sublimation, fusion and vaporization and phases of substance meet each other.
Q3. What is the use of steam table?
Ans. 1. Using calculations and experiments, it is possible to determine various properties of steam, such as its saturation temperature (or boiling temperature), pressure, enthalpies of water, evaporation, dry saturated steam, and superheated steam, as well as their respective specific volumes and entropies. Steam tables are lists of the properties of steam that are presented in a tabular format.
2. Saturated liquid and dry steam specific volume, enthalpy, and entropy values are provided in the steam tables and are tabulated against pressure (p) or the appropriate saturation temperature (Ts).
3. Steam tables provide quick discovery of the fundamental properties of steam given a set of parameters.
4. Parameters for intermediate values of pressure and temperature, which are not given in the steam tables, can be found by using interpolation method.
5. Important aspects on the application of steam tables :
a. Values of various properties have been given for one kg of water and one kg of steam (i.e., per kg basis).
b. Datum taken for calculation of enthalpy and entropy is 0 °C.
c. At lower and moderate pressures specific volume ‘vf’ of water can be neglected compared to specific volume ‘vg‘ of steam but at higher values of pressure, specific volume of water cannot be neglected.
d. Steam tables give the properties for liquids and dry steam and not wet steam.
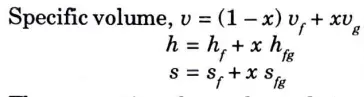
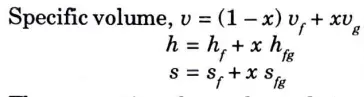
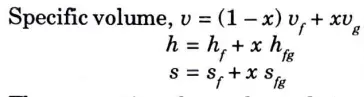
e. The properties of superheated steam are tabulated separately.
Q4. Write down the analysis of Rankine cycle.
Ans. A. Analysis of Rankine Cycle :
1. Let’s consider one kg of steam flow in the cycle. Apply steady flow energy equation (SFEE) to various processes discussed earlier :
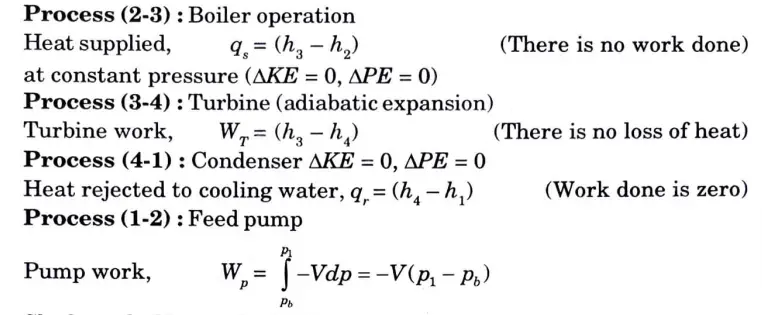


2. Shaft work (Net work done),



B. Efficiency of Rankine cycle:
1.
2. Since compression process in the pump is carried out with liquid only for which specific volume is very small, therefore pump work (h2 – h1) is quite small as compared to turbine work (WT) hence can be neglected.
Rankine cycle efficiency now becomes without pump work,
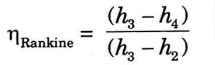
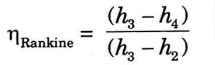
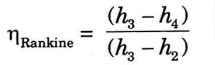
3. Rankine cycle on (p-V) and (T-s) diagram is shown in Fig.
Q5. Solve this Numerical
Question: A mixture of dry air and water vapour is at a temperature of 22 °C under a total pressure of 730 mm Hg. The dew point temperature is 15 °C. Find :
i. Partial pressure of water vapour;
ii. Relative humidity;
iii. Specific humidity;
iv. Enthalpy of air per kg of dry air;
v. Specific volume of air per kg of dry air.
Ans.
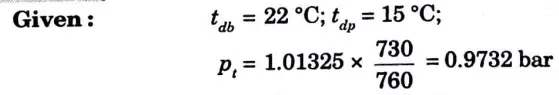
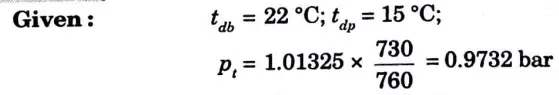
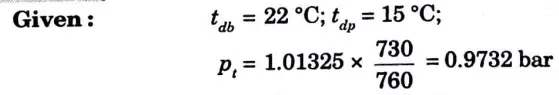
i. From steam tables partial pressure of water vapour at 15 °C dew point temperature,
Pv= 0.017 bar
ii. Saturation pressure of water vapour at 22 °C DBT,
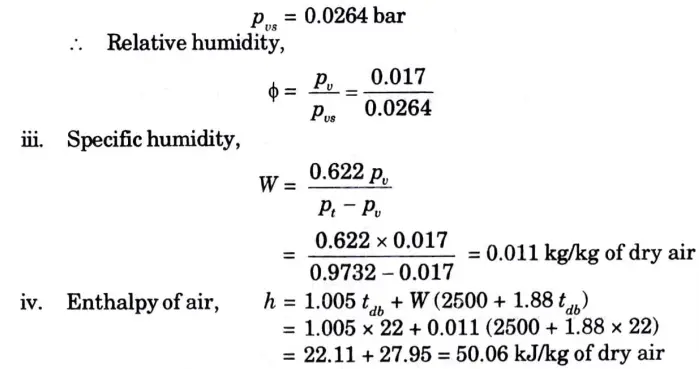


v. Specific volume of air is equal to the volume of 1 kg of dry air or 0.011 kg of water vapour. Based on dry air part
Q6. Solve this Numerical
Question: In laboratory test, a psychrometer recorded dry bulb temperature as 33 •c and wet bulb temperature 28 •c. Calculate :
- i. Vapour pressure;
- ii. Relative humidity;
- iii. Specific humidity;
- iv. Degree of saturation;
- v. Dew point temperature;
- vi. Enthalpy of mixture.
Barometric pressure = 1.0132 bar.
Ans.
Given:
From steam table, corresponding to 28 °C,
i. Vapour pressure is given by the relation :



Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
Thermodynamics Quantum, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Important Unit-1 | Unit-1 |
| Important Unit-2 | Unit-2 |
| Important Unit-3 | Unit-3 |
| Important Unit-4 | Unit-4 |
| Important Unit-5 | Unit-5 |
| Question paper – 2021-22 | 2021-22 |
Thermodynamics Quantum PDF: | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One VIew) | Student Result |

3 thoughts on “Unit 4: Steam and Rankine Cycle in Thermodynamics”