You will learn about the Second Law of Thermodynamics in Mechanical Engineering through the key questions in this course. Study heat engines, gain knowledge of system efficiency, and apply the concepts of the Second Law to actual engineering challenges.
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For Thermodynamics: *Unit-01 *Unit-02 *Unit-03 *Unit-04 *Unit-05 *Short-Q/Ans *Question-Paper with solution 21-22
Q1. What is heat reservoir? Also discuss about heat engine.
Ans. A. Heat Reservoir:
- 1. A body with a particularly high heat capacity is referred to as a heat reservoir because heat can be moved to and from it without causing any change in temperature.
- 2. A high temperature reservoir is the term used to describe such a thing.
- 3. It is regarded as a heat source if heat is transferred from it.
- 4. A low temperature reservoir is a body that is at a low temperature.
- 5. Something is a heat sink if heat is transferred to it.
- 6. A heat reservoir is a closed system that has no work interaction by definition.
- 7. The environment is the biggest heat reservoir that doesn’t change temperature and is typically used as a heat sink but can also act as a heat source on occasion.
- 8. The mediums in the environment which are used as such are generally the following:
- a. Atmosphere air,
- b. Ocean, river or well water, and
- c. Ground.
- 9. The characteristics which remains constant for a heat reservoir is its temperature. Hence, a heat reservoir is characterized by its temperature.
B. Heat Engine:
1. A heat engine is a thermodynamic system operating in a cycle to which net positive amount of heat is added, and from which net positive amount of work is obtained.
Q2. Define second law of thermodynamics.
Ans. 1. On the basis of limitations of first law of thermodynamics, we have two statements of second law of thermodynamics which are as follows:
A. Kelvin-Planck Statement: It is impossible to build a heat engine that runs in a cycle and generates no effects other than work output and heat exchange with a single heat reservoir, claims this statement.
B. Clausius Statement: 1. This statement asserts that “it is impossible to build a machine that functions in a cycle and generates no effect other than the transfer of heat from one system at high temperature to another.”
2. According to Clausius, heat cannot move by itself from an area of low temperature to a region of high temperature without the assistance of outside effort.
3. Heat engines are affected by the Kelvin-Planck and Clausius statements, but heat pumps and refrigerators are covered by the Clausius claims. Both of the second law of thermodynamics’ claims are contradictory and lack a corresponding mathematical proof.
Q3. A reversible heat engine operates between two reservoirs at 827 ℃ and 27 ℃. Engine drives a Carnot refrigerator maintaining -13 ℃ and rejecting heat to reservoir at 27 ℃. Heat input to the engine is 2000 kj and the net work available is 300 kj. Determine the heat transferred to refrigerator and total heat rejected to reservoir at 27 ℃.
Ans.



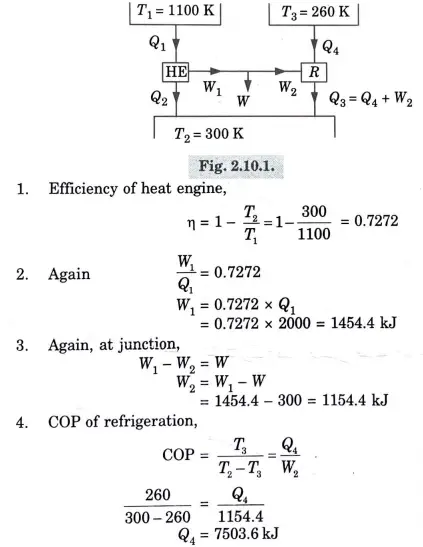


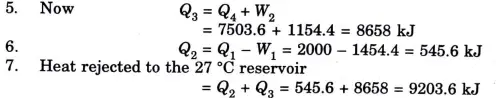
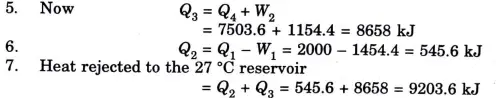
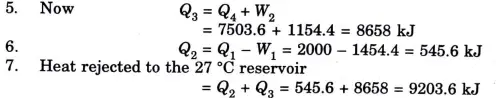
Q4. Why Carnot cycle is a theoretical cycle?
Ans. Carnot Cycle is a Theoretical Cycle: Carnot cycle cannot be used in a practical engine because the following reasons:
- 1. In order to ensure that the temperature stays constant during an isothermal (expansion or compression) process, the piston must move very slowly.
- 2. Due to the limited time available, adiabatic processes can only be achieved if the piston moves very quickly, making it possible to achieve negligible heat transfer between the system and its surroundings. The piston in a Carnot cycle must travel very slowly for a portion of the stroke and very quickly for the remainder because isothermal and adiabatic processes occur during the same stroke. From a kinematic standpoint, this kind of variance in piston movement within the same stroke is not possible.
- 3. It is impossible to achieve a frictionless process.
- 4. It is not possible to transfer heat without finite difference of temperature (according to second law of thermodynamics).
- 5. Real process can never be reversible process.
- 6. The atmosphere can be thought of as an infinitely huge sink at constant temperature, but a massive heat source that will deliver heat without causing a change in temperature can never be created.
- 7. The aforementioned objections explain why a Carnot cycle cannot be used in a real engine.
Q5. Solve the Numerical:
Question: What is perpetual motion machine of second type? A gas of mass 1.5 kg undergoes a quasi-static expansion which follows a relationship p = a +bV where a and b are constants. The initial and final pressures are 1000 kPa and 200 kPa respectively and the corresponding volumes are 0.20 m3 and 1.20 m3. The specific internal energy of the gas is given by the relation.
u = 1.5 pv – 85 kJ/kg
Where p is in kPa and v is in m3/kg\. Calculate the net heat transfer and the maximum internal energy of the gas attained during expansion.
Ans. A. Perpetual Motion Machine of Second Kind (PMM-2):
1. A device or machine which violates the second law of thermodynamics is known as perpetual motion machine of second kind (PMM-2).
2. A PMM-2 will exchange heat from a single thermal (or heat) reservoir and produce equal amount of work energy.
3. If heat rejection Q2 to the heat reservoir at T2 is zero, then



4. This is a violation of Kelvin-Planck statement of second law of thermodynamics. So it is impossible to construct a perpetual motion machine of second kind.
B. Numerical:



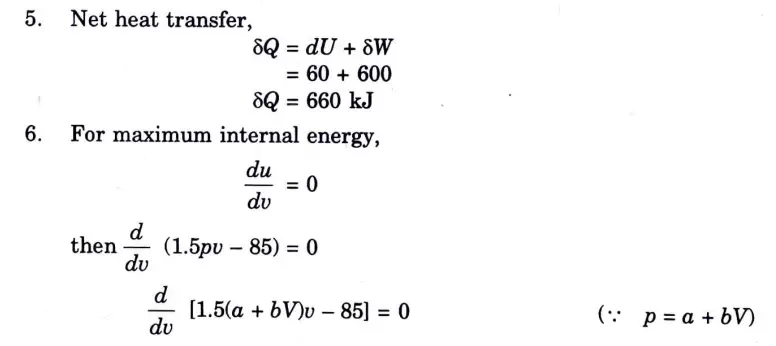
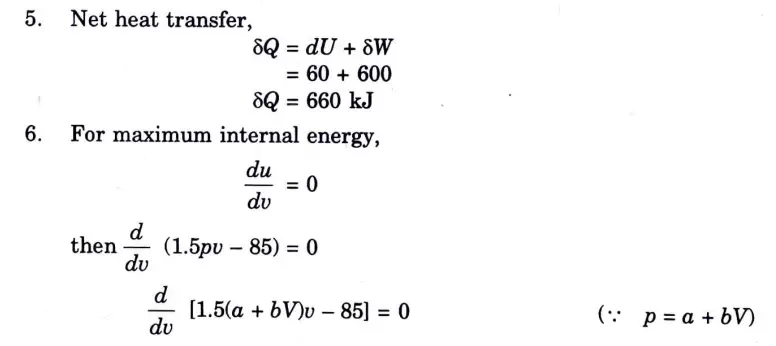
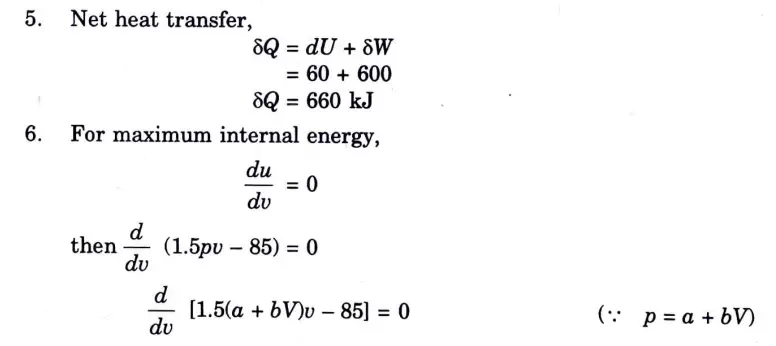
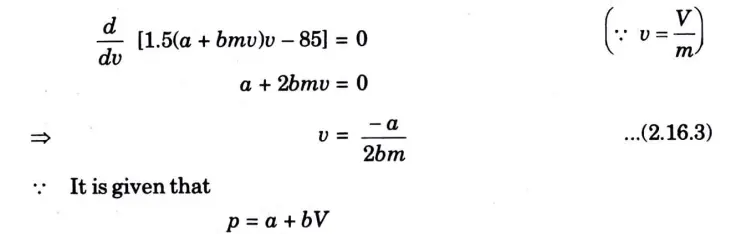
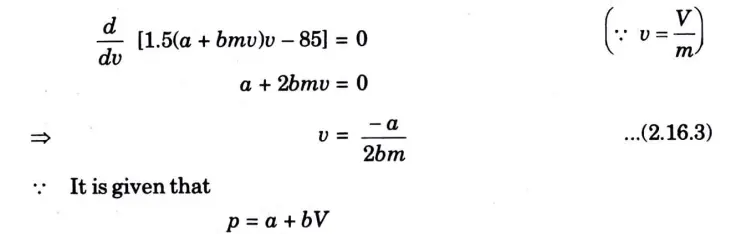
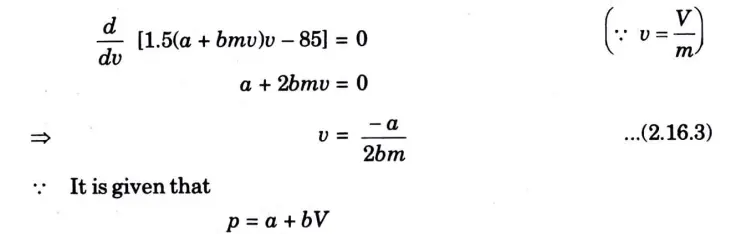
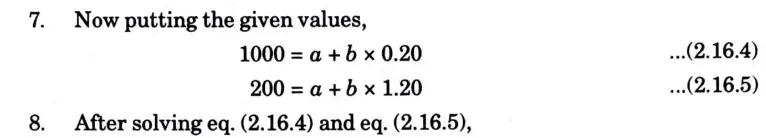
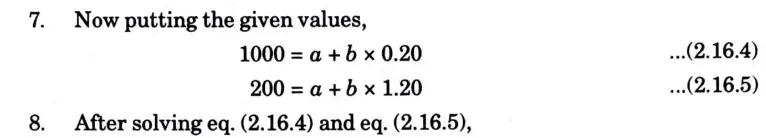
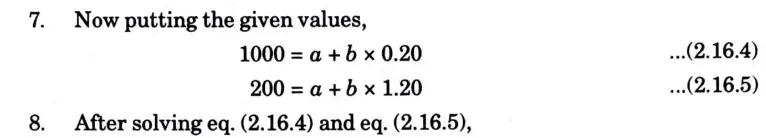
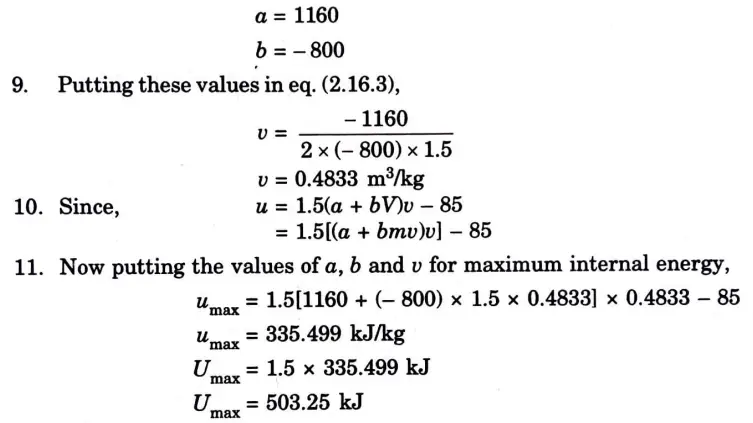
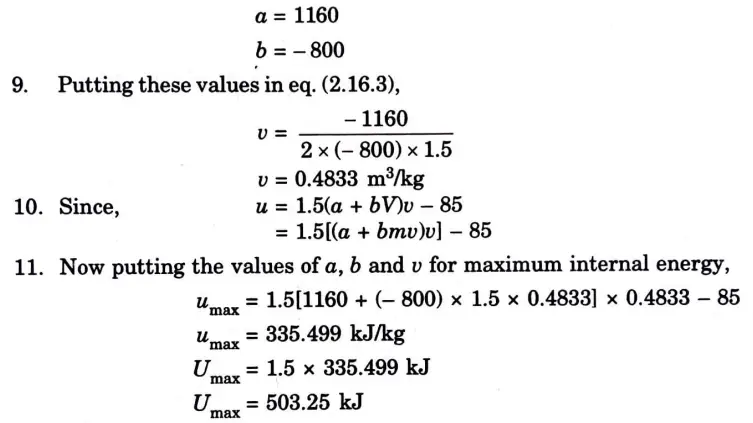
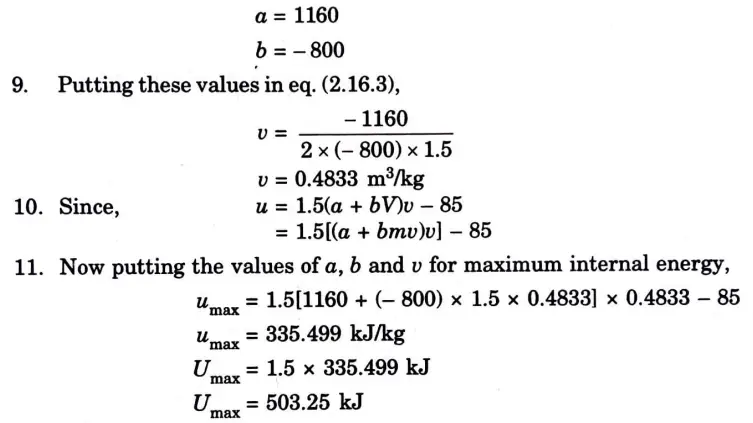
Q6. Define entropy. Prove that entropy is a point function.
Ans. A. Entropy:
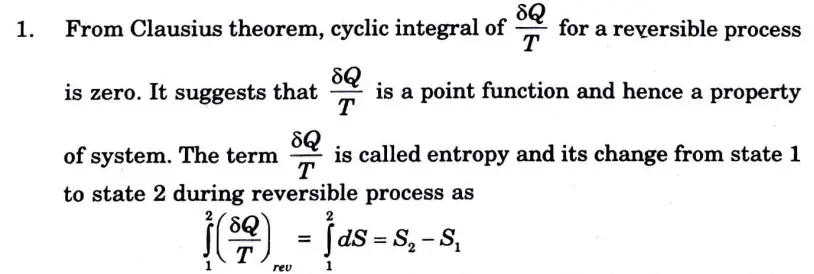


2. The degree of unpredictability among the molecules making up a system is measured by entropy. The increase in entropy is greater the more disorder there is.
3. In other words, entropy is a function of heat quantity that indicates the likelihood that heat could be converted into work. Entropy of the system increases with heat addition and lowers with heat rejection.
B. Entropy is a point function :
1. Consider a system taken from state ‘1’ to ‘2’ via A and is brought back to initial state ‘1’ via Band via C.
2. From Clausius theorem, for cycle 1-A – 2 – B – 1
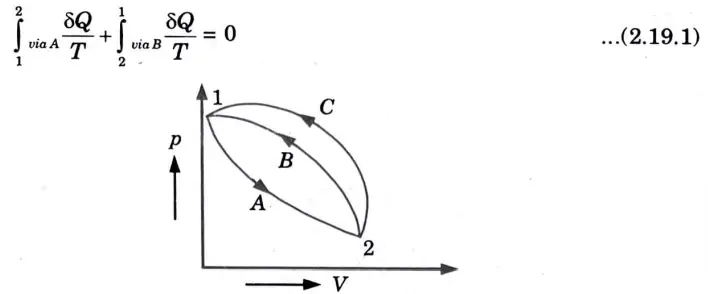


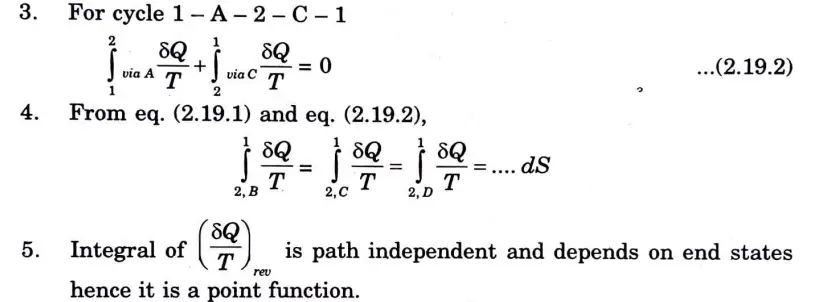





Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
Thermodynamics Quantum, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Important Unit-1 | Unit-1 |
| Important Unit-2 | Unit-2 |
| Important Unit-3 | Unit-3 |
| Important Unit-4 | Unit-4 |
| Important Unit-5 | Unit-5 |
| Question paper – 2021-22 | 2021-22 |
Thermodynamics Quantum PDF: | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One VIew) | Student Result |

5 thoughts on “Unit 02 Second Law of Thermodynamics in Mechanical Engineering”