Table Of Contents
We’re here to look at the most essential questions and notes regarding Air and Noise Pollution Control that may appear in your upcoming exams, such as Btech, MCA, and others. Unit-2 Chemistry and Dispersion of Air Pollution
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well.
Important Questions For Air and Noise Pollution Control:
*Quantum *B.tech-Syllabus
*Circulars *B.tech AKTU RESULT
* Btech 3rd Year * Aktu Solved Question Paper
Q1. Describe the air pollution chemistry.
Ans. Air Pollution Chemistry:
- 1. Some air pollutants that are released into the atmosphere as a result of human activity directly endanger the environment and human health.
- 2. These principal pollutants come from road vehicle exhausts and include lead, nitrogen oxides, particulate matter, carbon monoxide, and particulate matter.
- 3. Yet, secondary pollutants many of which have the potential to be even more dangerous than their precursors are created when primary pollutants through a complicated series of chemical reactions in the atmosphere.
- 4. The secondary pollutants are frequently referred to as photochemical pollutants because sunlight is largely responsible for the pollutant chemistry.
- 5. A well-known secondary photochemical pollutant is ozone (O3). Its formation results from the sunlight-initiated oxidation (reaction with oxygen) of volatile organic compounds (VOCs) such as benzene (C6H6) in the presence of nitrogen oxides (NOx), mostly nitric oxide (NO) and nitrogen dioxide (NO2).
- 6. Once formed, ozone is scavenged by NO, and in the absence of other competing reactions, a “photostationary state” is formed where concentrations of NO, NO2 and O3 are all inter-related.
- 7. Ozone scavenging by NO is less effective in rural locations distant from significant NO sources, such as urban road transportation, and as a result, ozone concentrations in the atmosphere are higher.
Q2. Describe the atmospheric temperature lapse rate and stability.
Ans. Atmospheric Temperature Lapse Rate:
- 1. The deposition of pollution will be at its highest when the air is moving at the slowest possible speed or is essentially stagnant.
- 2. Pollution levels will be low when the air is turbulent.
- 3. Instead of being observed by observatories, the vertical temperature profile is used to determine turbulence.
- 4. It has been recognised that the tendency of the atmosphere to resist or promote vertical motion, or to suppress or intensify already-existing turbulence, is a sign of atmospheric stability.
- 5. As the atmosphere is cooler at higher altitudes, normally the reduces at a temperature rate of 1°C per 100m height.
- 6. The term “adiabatic lapse rate” refers to this drop in temperature.
- 7. Yet, in the majority of situations, there is a reduction in temperature but a lower adiabatic lapse rate.
- 8. This type of shift is referred to as a sub adiabatic rate, and the atmosphere is seen to remain stable.
- 9. In these circumstances, the mixing of the occurs gradually. pollutant dilution and pollution.
- 10. The atmosphere is in an unstable state when the adiabatic rate of temperature decrease with height exceeds the rate, which is known as the super-adiabatic lapse rate.
- 11. These circumstances are ideal for blending and diluting contaminants.
- 12. The neutral state, also known as the adiabatic lapse rate, is characterized by cloudy days and nights.
- 13. An inversion occurs when the temperature rises with height sometimes.
- 14. If the conditions are met, the contaminants cannot diffuse and create a thick layer at the tops.
- 15. Pollutant emissions take place near or on the earth’s surface. Yet, the depth of the layer that they agitated or diffused varied across time and space.
- 16. Maximum mixing depth is the altitude above the atmosphere’s surface at which the adiabatic lapse rate crosses the measured vertical temperature profile (MMD).
- 17. Ground level concentration will be relatively high when the mixing height is low but still above plume height because the pollutants are then unable to disperse in the upper atmosphere.
Q3. What are the impact of atmospheric pressure on dispersion of air pollutants?
Ans. Impact ofAtmospheric Pressure on Dispersion of Air Pollutants:
- 1. However the development of an area’s high pressure system could lead to major air pollution issues if it lasts for several days due to the development of inversion conditions.
- 2. As a result, high pressure systems (anticyclones), which are accompanied by sunny sky, light winds, and stable atmospheric conditions, may be detrimental to the dispersion of pollutants.
- 3. In contrast, low pressure systems (cyclones), which are linked to extremely erratic atmospheric conditions, typically cause good mixing and quick dispersion of contaminants.
- 4. As a result, the dispersion of pollutants is improved by such cyclonic air conditions, which are frequently accompanied by rain and storms.
- 5. Nevertheless, opposing affects result when a warm front passes over a low pressure cell.
- 6. Storm activity along the warm front’s leading edge will initially lessen the pollutant load, but as the front grows stronger, more stable conditions will arise, increasing the potential for air pollution.
Q4. Discuss the impact of moisture and precipitation on dispersion of air pollutants.
Ans. Impact of Moisture and Precipitation on Dispersion of Air Pollutants :
- 1. The amount and type of moisture in the atmosphere can have a significant impact on the air quality in a given area.
- 2. The presence of water vapour (humidity) in the air has an impact on air quality, principally by impeding heat radiation reflected off the surface and blocking solar radiation from reaching the ground.
- 3. Humidity amplifies the corrosive effects of air pollutants on the Earth and causes fog to form.
- 4. Excessive atmospheric moisture will eventually result in rain, which helps to improve the quality of the surrounding air by washing down contaminants to the Ground, where they will eventually be drained out by rain-run off.
- 5. The process of removal of atmospheric SO2 through rain, may, however, cause problems due to reaction of SO2 with water, forming H2SO3 or H2SO4 leading to fall of acid rain, which increases the rate of corrosion where air pollutants are present and in addition, decreases the pH of rivers and streams, adversely influencing the algae and plant life of such water bodies.
Q5. How do winds impact the dispersion of pollutants into the ambient air environment?
Ans. Impact of Winds on Dispersion of Pollutants:
- 1. Wind is the name for the moving air. Such air movement is brought about by the uneven distribution of atmospheric temperature over the pressure surface of the Earth and is greatly impacted by the earth’s rotation.
- 2. Winds always blow from high to low pressure areas, but the coriolis force tends to divert air currents from these predictable patterns.
- 3. Local and regional topographical features can also have an impact on the direction and speed of winds.
- 4. The flow of sea breezes from the sea to land during the day and the flow of land breezes from the land to the sea during the evenings after sundown may both be caused by the Earth’s faster heating and cooling than the nearby sea.
- 5. Problems with air pollution may also be caused by such a wind pattern.
- 6. Winds are often gusty and changeable in the friction layer at the Earth’s surface, mostly because of locally produced mechanical or thermal turbulence.
- 7. Wind speed is usually measured by an anemometer at a Knowing height, say Z0 the wind velocity (u0) at anemometer height (Z0), we can work out the velocity u at any other height Z by using the formula
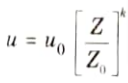
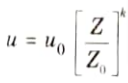
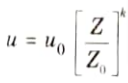
Where, k is a constant 1/9 for large lapse rates, and 1/3 for marked inversions, average normal value being 1/7.
- 8. The drift and diffusion of toxic gases and particulate emissions from autos, factories, etc. that are released close to the ground levels are principally controlled by the direction and speed of surface winds.
- 9. Pollutants would be moved away from the source more quickly the faster the wind was blowing at or near the point of emission.
- 10. The contaminants will not be present at the same concentration since they will be rapidly diluted by expanding air volumes.
Q6. Describe turbulence effects on air pollutions.
Ans.
- 1. There are basically two different causes of turbulent eddies:
- i. Mechanical turbulence.
- ii. Convective turbulence.
- 2. In any given atmospheric situation, both of them are typically present, although mechanical or convective turbulence predominates over the other.
- 3 Mechanical Turbulence:
- i. Physical barriers to regular flow, such as mountains, structures, trees, etc., are to blame.
- ii. The amount of mechanical turbulence is influenced by the obstacles’ roughness and wind speed.
- 4. Convective Turbulence:
- i. It happens as a result of various air masses and surface heating and cooling.
- ii. The air pollution turbulence increases with the difference in atmospheric temperature.
- 5. Atmospheric eddies break apart atmospheric parcels, allowing polluted air to inhabit progressively bigger volumes of space at lower and lower concentrations.
- 6. Thus the level of turbulence in the atmosphere determines its dispersive ability.



Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
Air and Noise Pollution Control Btech Quantum PDF, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Question paper – 2021-22 | 2021-22 |
Air and Noise Pollution Control Quantum PDF | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One View) | Student Result |
