B.Tech AKTU Quantum Book will take you on a journey through the world of Heat and Mass Transfer. To do well in this subject, find important notes, repeated questions, and critical insights. Unit-1 Introduction to Heat Transfer
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For Heat and Mass Transfer: *Quantum *B.tech-Syllabus *Circulars *B.tech AKTU RESULT * Btech 3rd Year * Aktu Solved Question Paper
Q1. Define thermal conductivity. Discuss the effect of temperature on thermal conductivity.
Ans. A. Thermal Conductivity:
- 1. When the temperature difference between the faces causing heat flow is unity, the amount of heat carried through a body of unit area and unit thickness in unit time.
- 2. The unit of thermal conductivity is W/mK or W/m °C.
B. Effect of Temperature on Thermal Conductivity:
- 1. The thermal conductivity of most metals diminishes as temperature rises.
- 2. As the temperature rises, the density of the solid reduces, and therefore the thermal conductivity.
- 3. Thermal conductivity levels in most liquids tend to decrease with temperature due to density decrease and temperature increase.
- 4. Nevertheless, thermal conductivity increases with temperature in the case of gases because thermal conductivity is directly proportional to the mean free path of the molecules.
- 5. As a result, gases with higher molecular weights have lower thermal conductivity than gases with lower molecular weights.
- 6. The dependence of thermal conductivity on temperature is as given below,



- 7. Thermal conductivity of porous material depends upon the type of gas or liquid present in the voids.
Q2. What are the mechanisms of heat transfer ? How are they distinguished from each other ?
Ans. A. Mechanisms of Heat Transfer: Following are the three modes of heat transfer:
a. Mechanism of Heat Transfer through Conduction:
- 1. Thermal conduction is a heat propagation mechanism that occurs within a medium or between various mediums in direct physical contact from an area of higher temperature to a region of lower temperature.
- 2. There is no movement of macroscopic portions of matter relative to one another in conduction.
- 3. The conduction is done by:
- i. As a result of random molecular motion, i.e., vibration of molecules about their equilibrium position, the idea is known as micro forum or heat transmission and is sometimes referred to as energy diffusion.
- ii. Thermal energy can be conveyed using electrons that are free to flow via the material’s lattice structure.


b. Mechanism of Heat Transfer by Convection:
- 1. Thermal convection is an energy transmission process influenced by the circulation or mixing of a fluid media.
- 2. Convection is only conceivable in a fluid medium and is intimately related to medium transport.
- 3. The mixing motion of the fluid greatly influences the effectiveness of heat transfer by convection.
- 4. There are basically two types of convection:
- i. Natural or free convection, and
- ii. Forced convection.
- 5. Convective heat transfer is prescribed by Newton’s law of cooling i.e.,



c. Mechanism of Heat Transfer by Radiation:
- 1. The mechanism of heat transfer by radiation consists of three distinct phase:
- i. Conversion of the hot source’s thermal energy into an electromagnetic wave.
- ii. Wave motion passing through intervening space.
- iii Wave to heat transformation.
- 2. Radiation heat transfer is governed by Stefan-Boltzmann law :



B. Difference between Conduction, Convection and Radiation:
| S. No. | Conduction | Convection | Radiation |
| 1. | Heat moves from the hot end to the cold end. Particles in the media just fluctuate but do not move. | Each particle absorbing heat is mobile. | Heat travels in the form of electromagnetic waves without any intervening medium. |
| 2. | Conduction requires a medium. | Medium is necessary for convection. | Radiation does not require a medium. |
| 3. | It is a slow process. | It is also a slow process. | It is a very fast process. |
| 4. | Path of heat flow may be zig-zag. | Path may be zig-zag or curved. | Path is a straight line. |
| 5. | Conduction takes place in solids. | Convection takes place in fluids. | Radiation occurs in both gaseous and transparent mediums. |
| 6. | The temperature of the medium through which heat passes rises. | The temperature of the medium rises throughout this phase as well. | The medium’s temperature remains unchanged. |
Q3. What do you mean by analogous system ? Explain the thermal resistance in case of conduction, convection and radiation.
Ans. A. Analogous System:
- 1. Two physical systems are considered to be comparable if they are represented by similar equations and have similar boundary conditions.
- 2. The heat transmission mechanism is analogous to the flow of electricity in an electrical system.
B. Thermal Resistance :
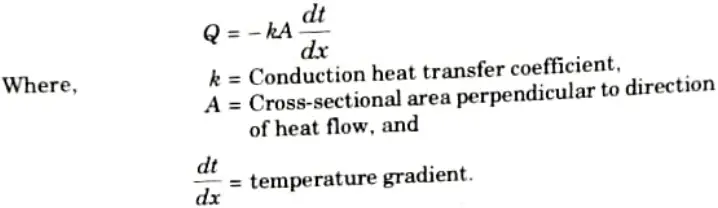
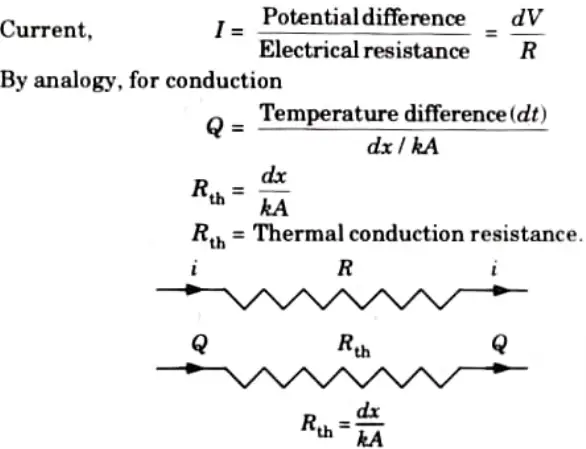
i. For Conduction:
As per Ohm’s law:


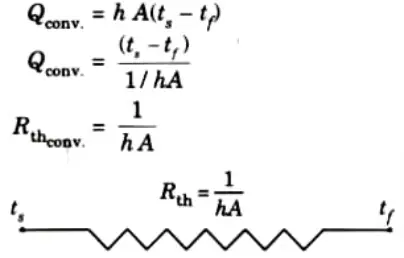
ii. For Convection: Convection heat transfer is given by Newton’s law of cooling i.e.,


iii. For Radiation: Radiation heat transfer is governed by Stefan Boltzmann law i.e.,
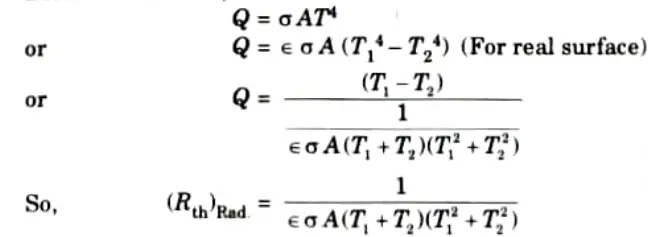
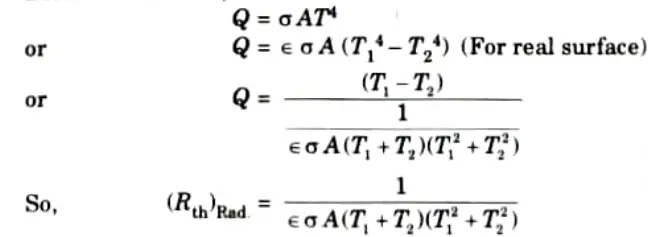
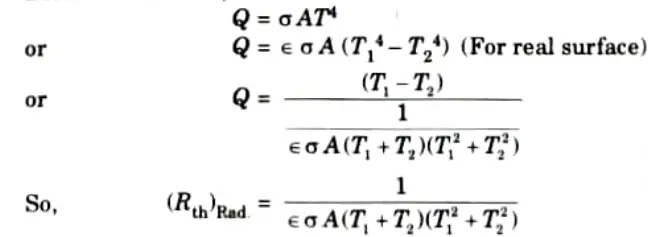
Q4. Explain thermal contact resistance.
Ans. 1. The contact surfaces only touch in discrete places in real systems because of surface roughness and vacant areas. As a result, relative to the geometric face area, the area available for heat movement at the interface will be tiny. A significant resistance to heat transfer arises at the interface as a result of this smaller area and the presence of air spaces. Thermal contact resistance is the name given to this resistance.
Mathematically,
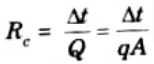
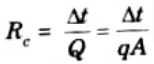
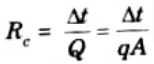
2. It causes temperature drop between two materials at the interface.
Q5. Derive the general heat conduction equation in cylindrical coordinates.
Ans. 1. Consider an elemental volume having the coordinate (r, 𝛟, z) for three dimensional heat conduction analysis as shown in Fig.
2. The volume of the element,
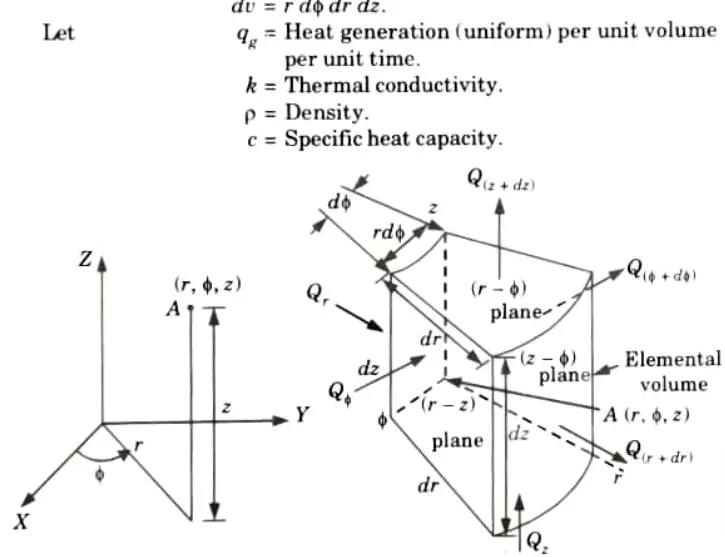


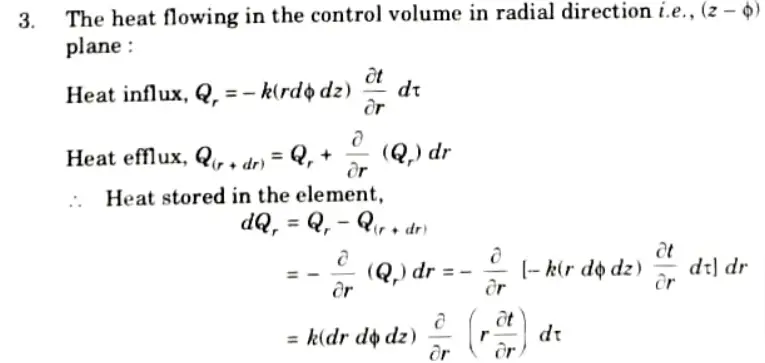





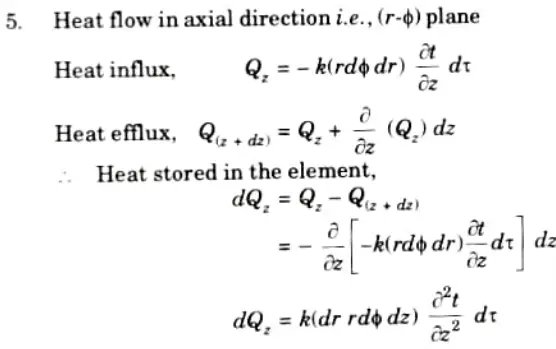


6. Net heat stored in the element
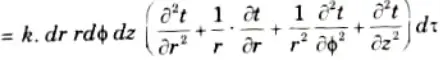
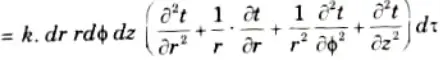
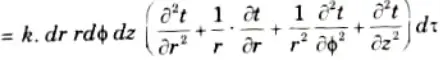
7. Heat generated within the control volume,
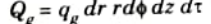
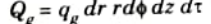
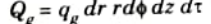
8. The increase in thermal energy in the element equal to
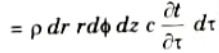
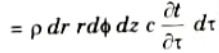
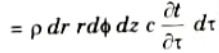
9. From the principle of conservation of energy:
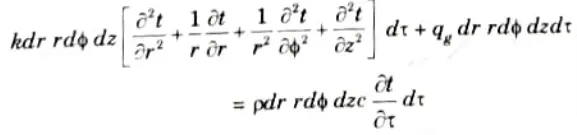
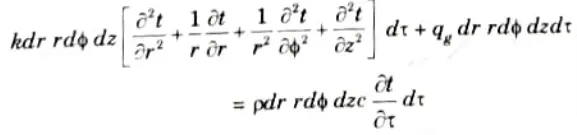
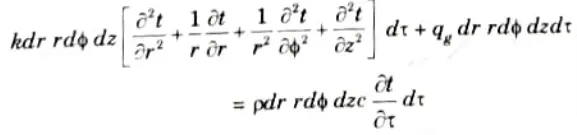
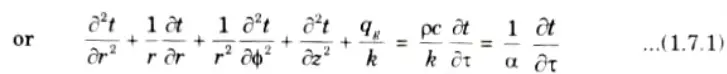
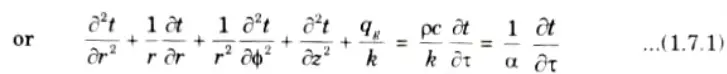
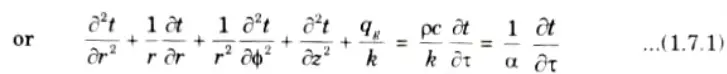
This is the general heat conduction equation in cylindrical coordinates.
1. For steady-state, unidirectional heat flow in the radial direction, and with no internal heat generation, eq. (1.7.1) reduces to
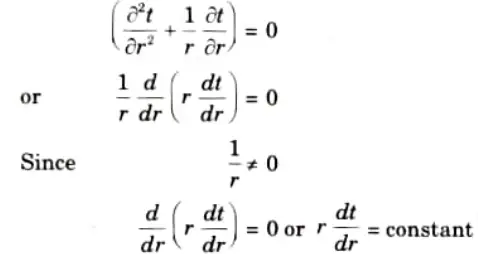
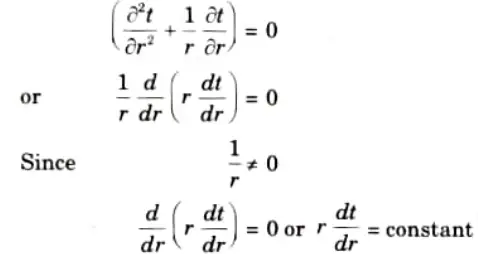
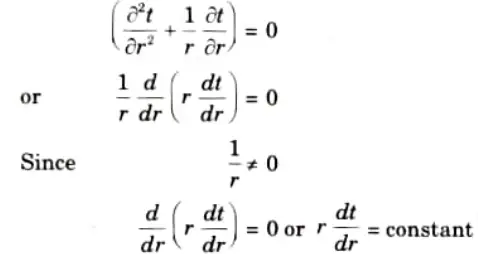
Q6. Define initial conditions and boundary conditions. Explain the different types of boundary conditions applied to heat conduction problem.
Ans. A. Initial Conditions:
1. The initial conditions, which are only required for transient (time-dependent) problems, explain the temperature distribution in a medium at the beginning of time.
2. The initial conditions can be expressed as
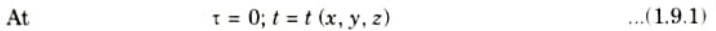
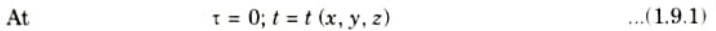
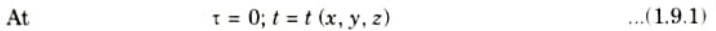
3. For a uniform initial temperature distribution, a simple but typical form of the above identity can be recast as
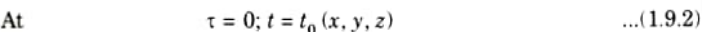
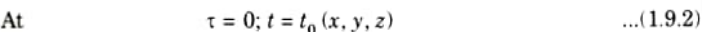
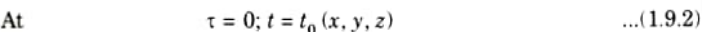
B. Boundary Conditions:
1. The temperature or heat flow at the body’s surface is specified by the boundary conditions, which are physical conditions that exist at the medium’s boundaries.
C. Types of Boundary Conditions:
The different types of boundary conditions applied to heat conduction problems are as follows:
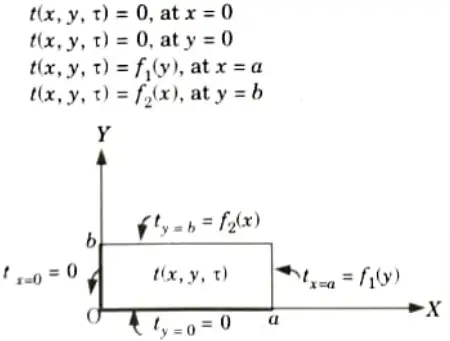
a. Boundary Condition of First Kind (Prescribed Surface Temperature) :
1. The temperature distribution, ts is given at a boundary surface for each moment of time.
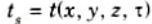
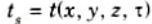
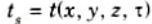
2. A typical example of boundary condition of the first kind for a slab is shown in Fig.


b. Boundary Condition of the Second Kind (Prescribed Heat Flux):
1. In this case, the heat flux at a boundary is prescribed.
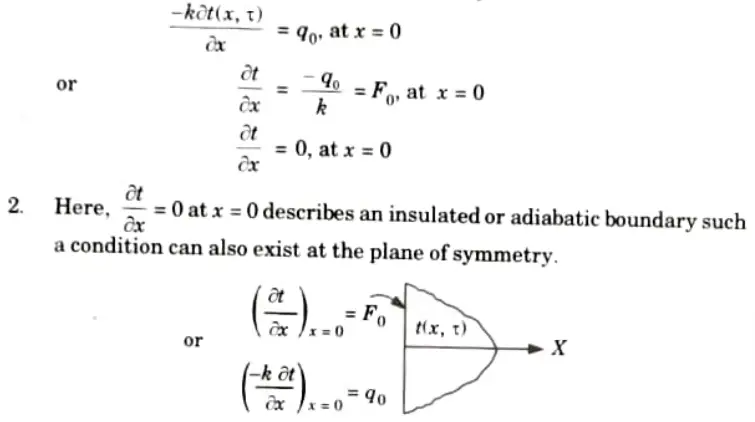


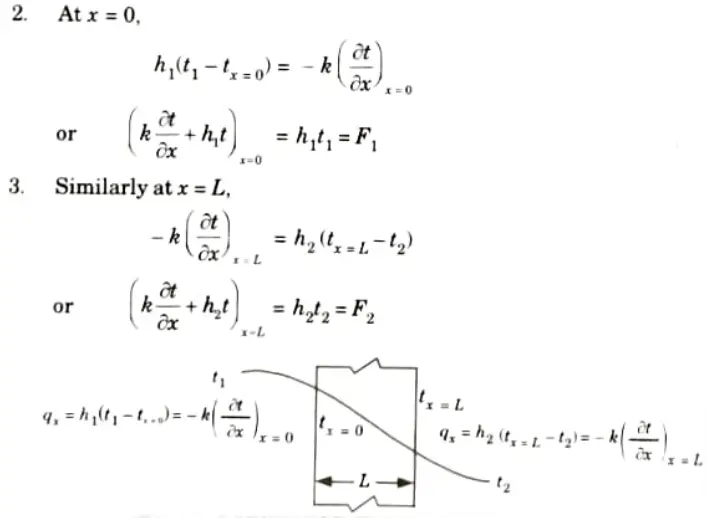
c. Boundary Condition of Third Kind (Convective Condition) :
1. When the rate of heat transfer from the surface via convection and that from the surface by conduction are equal at a solid boundary, this condition occurs.





Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
Heat and Mass Transfer Btech Quantum PDF, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Question paper – 2021-22 | 2021-22 |
Heat and Mass Transfer Quantum PDF | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One View) | Student Result |
