B.Tech AKTU Quantum Book will take you on a journey through the world of Heat and Mass Transfer. To achieve success in this subject, find important notes, repeated questions, and critical insights. Unit-5 Heat Exchanger
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For Heat and Mass Transfer: *Quantum *B.tech-Syllabus *Circulars *B.tech AKTU RESULT * Btech 3rd Year * Aktu Solved Question Paper
Q1. What do you mean by fouling in heat exchangers ? Briefly explain the fouling factor.
Ans. A. Fouling:
- 1. Fouling is the term used to describe the process of rust development and fluid impurity deposition.
- 2. As a result of these surface deposits, the heat exchanger’s performance eventually declines and thermal resistance rises.
B. Fouling Factor:
- 1 Since it is difficult to ascertain the thickness and thermal conductivity of the scale deposits, the effect of scale on heat flow is considered by specifying an equivalent scale heat transfer coefficient hs.
- 2. The reciprocal of scale heat transfer coefficient, hs is called the fouling factor, Rf



- 3. Fouling factors are determined experimentally by testing the heat exchanger in both the clean and dirty conditions. The fouling factor, Rf is thus defined as
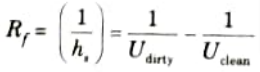
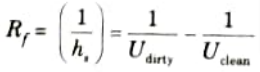
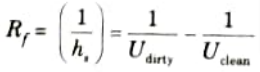
- 4. If hsi and hso be the heat transfer coefficients for the scale deposited on the inside and outside surfaces respectively, then the thermal resistance to scale formation on the inside surface (Rsi) and outside surface (Rso) are given by
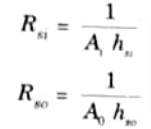
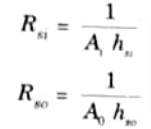
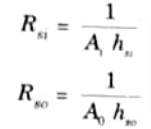
Q2. What do you understand by overall heat transfer coefficient ? Write its expression for plane wall and surfaces ?
Ans. A. Overall Heat Transfer Coefficient: It is a measurement of the amount of heat that is transferred over a given area during a given time at a given temperature differential between the bulk fluids on either side of the substance.
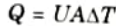
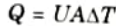
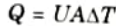
Where, U = Overall heat transfer coefficient.
B. Expression for Plane Wall and Cylindrical Surface:
1. The overall heat transfer coefficient for two fluids separated by a wall plane is given by :
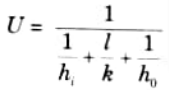
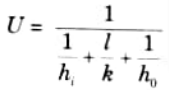
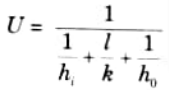
2. The overall heat transfer coefficient for fluids which are separated by a tube wall is given by:
i. For inner surface:
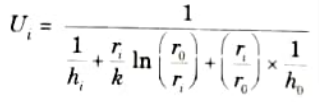
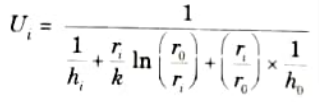
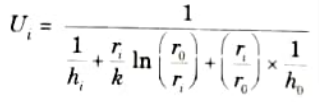
ii. For outer surface:
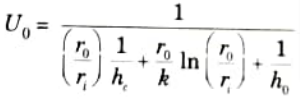
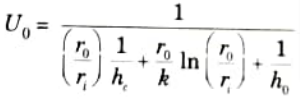
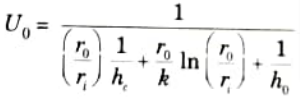
Q3. Under what conditions is the effectiveness – NTU method definitely preferred over the LMTD method in heat exchanger analysis ?
Ans.
- 1. The LMTD method has been employed in the thermal analysis of many types of heat exchangers.
- 2. When all of the terminal temperatures are known or can be easily determined, this method can be used to construct heat exchangers.
- 3. A problem develops if the fluid temperatures entering or leaving the heat exchanger are unknown.
- 4. This kind of circumstance arises while choosing a heat exchanger or when operating the exchanger outside of its intended parameters.
- 5. In these situations, it is recommended to use the effectiveness-NTU method, a completely distinct approach.
Q4. Explain condensation and classify the modes of condensation.
Ans. A. Condensation: By releasing its latent heat of vaporisation, it is a process by which a substance transitions from its vapour phase to its liquid phase.
B. Modes of Condensation: Following are the modes of condensation:
a. Filmwise Condensation:
- 1. In film condensation, the condensation liquid wet the surface and forms a liquid film.
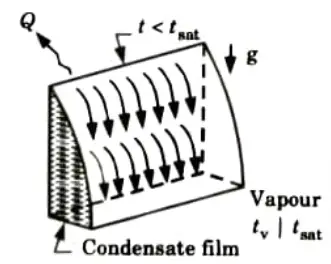


- 2. This liquid layer progressively builds up on the surface and acts as a barrier to the heat flow from the surface, preventing heat transmission from the surface to the condensing vapour.
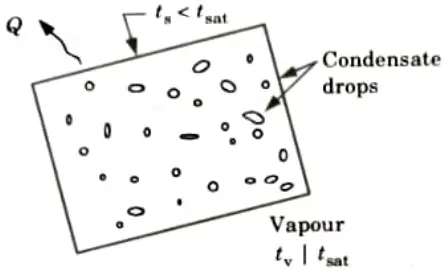
b. Dropwise Condensation:
- 1. Dropwise condensation is the process by which a vapour condenses into a variety of sized liquid droplets that randomly fall to the surface.
- 2. Since there is no insulating barrier of condensate liquid present during this condensation process, a wide area of solid surface is directly exposed to vapour, resulting in a higher heat transfer rate.


Q5. Discuss briefly the Fick’s law of diffusion.
Ans. 1. Fick’s law of diffusion states that the mass flux of species A per unit area is proportional to the concentration gradient.
2. So, diffusion rate of species A is given by,
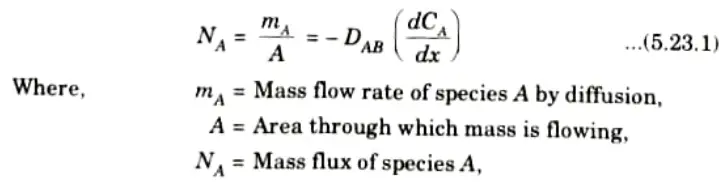





3. Similarly, diffusion rate for species Bis given by,
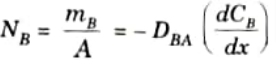
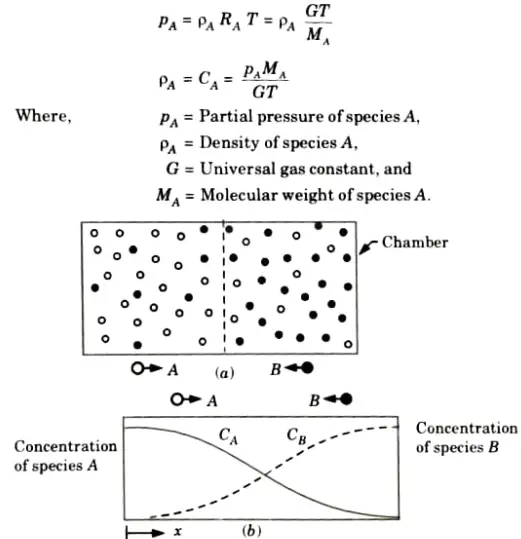
4. Fick’s law can be expressed in terms of partial pressure of species by using perfect gas equation.
For species A:


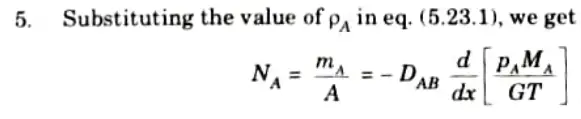
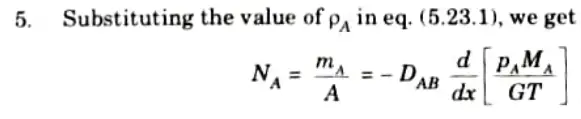
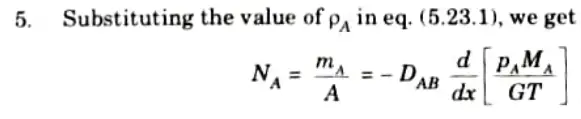
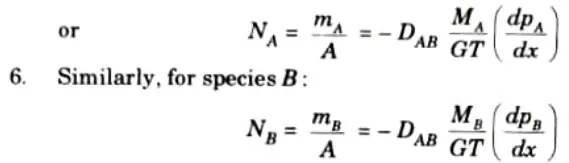
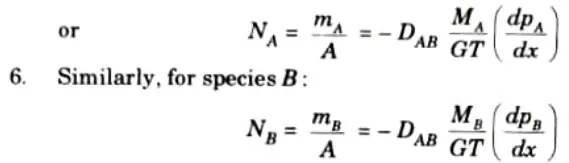
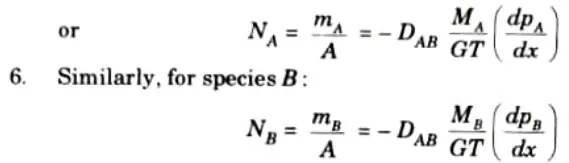
Q6. Explain boiling and discuss the various types of boiling.
Ans. A. Boiling:
- 1. It involves a phase transition from the liquid to the vapour state and is a convective heat transfer process.
- 2. It is also defined as evaporation at a solid-liquid surface. This is possible only when the temperature of the surface (ts) exceeds the saturation temperature corresponding to the liquid pressure (tsat).
- 3. Heat is transferred from the solid surface to the liquid according to the law
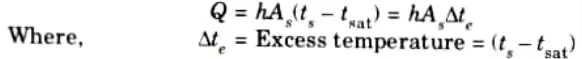
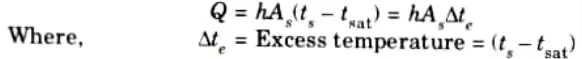
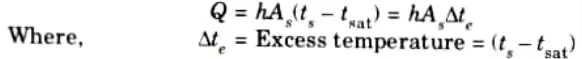
B. Types of Boiling: There are mainly four types of boiling :
- a. Saturated Boiling: As a liquid boils to a temperature over its saturation point, bubbles start to develop at the heating surface, move to the liquid’s top surface, and eventually escape the surface.
- b. Pool Boiling: This is the situation where the liquid is largely stationary above the heated surface and is only moving near the surface due to free convection and mixing brought on by bubble detachment and growth. Natural convection-based steam boilers are where it happens.
- c. Forced Convection Boiling: In forced convection boiling, external devices such as fans, pumps, etc. are used to cause fluid to move through the heated surface.
- d. Sub-cooled Boiling: In sub-cooled boiling, the liquid’s temperature is below its saturation point, boiling occurs close to the heated surface, and bubbles develop at the heated surface before dissipating after a little distance.



Important Question with solutions | AKTU Quantums | Syllabus | Short Questions
Heat and Mass Transfer Btech Quantum PDF, Syllabus, Important Questions
| Label | Link |
|---|---|
| Subject Syllabus | Syllabus |
| Short Questions | Short-question |
| Question paper – 2021-22 | 2021-22 |
Heat and Mass Transfer Quantum PDF | AKTU Quantum PDF:
| Quantum Series | Links |
| Quantum -2022-23 | 2022-23 |
AKTU Important Links | Btech Syllabus
| Link Name | Links |
|---|---|
| Btech AKTU Circulars | Links |
| Btech AKTU Syllabus | Links |
| Btech AKTU Student Dashboard | Student Dashboard |
| AKTU RESULT (One View) | Student Result |
