Hello and welcome to our blog! Through succinct notes on a question paper, investigate the realm of Air and Noise Pollution Control. Learn about fundamental topics such as common contaminants and the impacts of noise pollution. Don’t forget to check out Quantum Notes, where we explore the intriguing world of quantum mechanics. Join us on this educational adventure!
Dudes 🤔.. You want more useful details regarding this subject. Please keep in mind this as well. Important Questions For Air and Noise Pollution: *Quantum *B.tech-Syllabus *Circulars *B.tech AKTU RESULT * Btech 3rd Year
Section A : Short Question with Quantum PDF
a. Define lithosphere.
Ans. The lithosphere is the stiff, outermost shell on Earth. It is made up of the crust and the region of the upper mantle that exhibits elastic behaviour over timescales of at least a few tens of thousands of years.
b. What do you understand by hydrocarbon ?
Ans. A hydrocarbon is a class of organic chemicals made up of only the elements carbon (C) and hydrogen (H). The hydrogen atoms attach to the carbon atoms in a variety of ways to create the compound’s structural framework.
c. Define air pollution dispersion.
Ans. Pollutant dispersion is the transportation of aerial pollutants in the outdoor atmosphere after being emitted from the sources.
d. What do you know about lapse rate ?
Ans. The lapse rate is defined as the rate at which atmospheric temperature decreases with an increase in altitude. The term arises from the word lapse in the sense of a decrease or decline.
e. Enumerate ambient air quality standards adopted in India.
Ans. Euro VI – since 1st April 2020.
f. What are wet collectors ?
Ans.
- 1. Wet scrubbers are efficient devices for removing particles and gases from industrial exhausts.
- 2. In this device polluted air passes through absorbent liquid or water sprayed on the polluted air.
- 3. So these dirty particles of air stream contacts the liquid, the the liquid absorbs pollutants, in much the same way that rain droplets wash away strong odours on hot summer days.
g. Write method for removal of NOx from fuel gases.
Ans. The major technologies can be classified into different groups:
- (i) Nitrification,
- (ii) Denitrification,
- (iii) Microalgae,
- (iv) BioDeNOx,
- (v) Scrubbing,
- (vi) Adsorption,
- (vii) Selective non-catalytic reduction (SNCR),
- (viii) Selective catalytic reduction (SCR).
h. List any two advantages and disadvantages of scrubbers.
Ans.
A. Advantages of Scrubbers: Following are the advantages of scrubbers:
- 1. It is a very efficient process to separate dirt from ambient air.
- 2. It can handle flammable and explosive dusts with little risk.
B. Disadvantages of Scrubbers: Following are the disadvantages of scrubbers:
- 1. High potential for corrosion problems.
- 2. Disposal of waste sludge can be very expensive.
i. Define acoustic.
Ans. Acoustics is a branch of physics that studies sound. Acoustics is concerned with the production, control, transmission, reception, and effect of sound. Knowledge of this science is used to design and build theaters, cinemas, and other structures with proper acoustic conditions.
j. Write effect of noise pollution on the human health.
Ans. Following are the effects of noise pollution on human health:
- i. Noise pollution causes many mental, physical and physiological diseases in human beings and animals.
- ii. Health affect of noise include anxiety and stress reaction and in extreme cases fright.
- iii. The physiological manifestation are headaches, irritability and nervousness, feeling of fatigue and decreases work efficiency.
Section B : Important Long Question Air and Noise Pollution Control
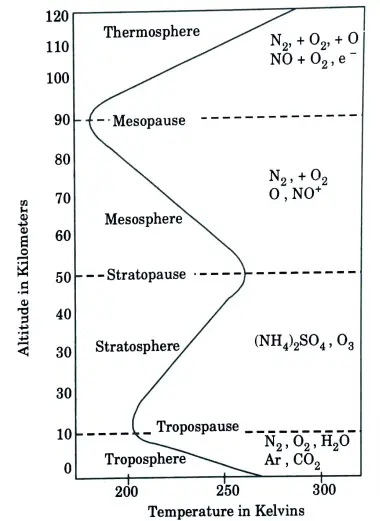
a. Define troposphere, stratosphere with respect to the ranges of altitude also mention the range of temperature and important gases found in each of these layers.
Ans. 1. Troposphere:
- i. The troposphere is the first layer of the Earth’s atmosphere.
- ii. All the events related to seasons take place in this sphere, such as formation of clouds, lightning.
Its height ranges from 8 km to 12 km, and it contains 3/4 of the atmosphere.
2. Stratosphere:
- i. The stratosphere is the second major layer of the Earth’s atmosphere.
- ii. The height of the stratosphere is about 50 km from the surface of the earth. We also call it protective shield.
- iii. The ozone layer is also available in it which protects from harmful ultraviolet rays.
- iv. This ozone layer is mostly harmed by chlorofluorocarbon (CFC).


b. How is maximum mixing depth (MMD) determined? Explain the significance of maximum mixing depth in air pollution control.
Ans. A. Maximum Mixing Depth (MMD):
- 1. The dispersion of pollutants in the lower atmosphere is greatly aided by the convective and turbulent mixing that takes place.
- 2. The vertical extent to which this mixing takes place depends on the environmental lapse rate which varies diurnally, from season to season and is also affected by topographical features.
- 3. The depth of the convective mixing layer in which vertical movement of pollutants is possible, is called the maximum mixing depth (MMD).
- 4. The maximum mixing depth (sometimes called the mixing height) is obtained by projecting the dry adiabatic lapse rate line to the point of intersection with the atmospheric temperature profile.
- 5. These profiles are usually measured at night or early in the morning.
- 6. An air parcel at a temperature warmer than the existing ground level temperature rises and cools according to adiabatic lapse rate.
- 7. The level where its temperature becomes equal to the air surrounding gives the MMD value.
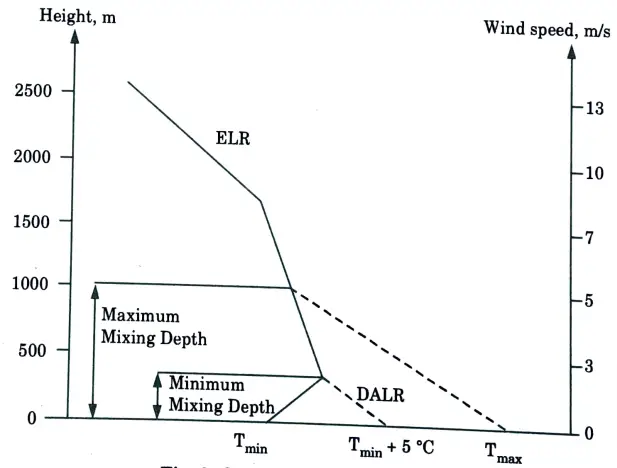


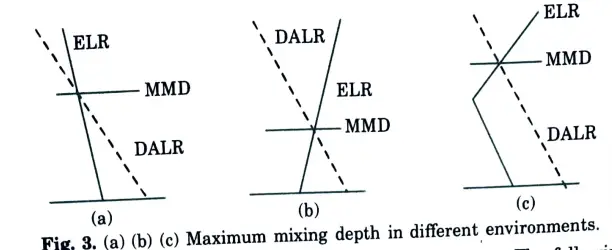


B. Determination of Maximum Mixing Height: The following steps can be used to determine the maximum mixing height for a day from a temperature profile:
- 1. Plot the temperature profile (ELR), if needed.
- 2. Plot the maximum surface temperature for the day on the graph for morning temperature profile.
- 3. Draw a dry adiabatic line (-1°C/100 m) from the point of maximum surface temperature to the point where it interests the morning temperature profile.
- 4. Read the corresponding height above ground at the point of intersection obtained in step 2. This is the maximum mixing height for the day.
c. Briefly describe the Guassian dispersion model for air pollutants ? Also write Holland’s equation for the calculation of plane height.
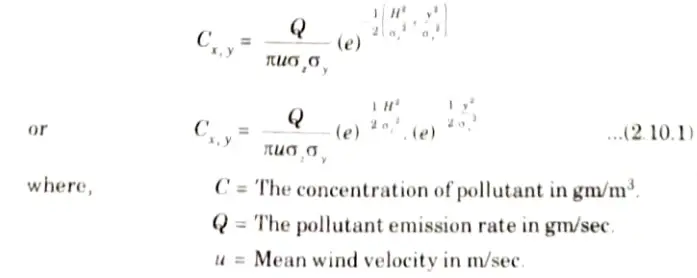
Ans. Guassian Dispersion Model:
- 1. The Gaussian plume model is the most common air pollution model for estimating concentrations from point sources downwind.
- 2. Employing a three-dimensional axis of downwind (x), crosswind (y), and vertical (z) with the origin at the effective height of emission, it assumes that the time-averaged plume concentrations from a continuously emitting plume, at each downwind distance, have independent Gaussian distributions both in the horizontal and the vertical.
- 3. Following are the assumptions of Gaussian model:
- i. Concentrations and emission rates are inversely proportional.
- ii. At the point of emission, pollutants are diluted by the wind at a rate inversely proportional to the wind speed, which is constant over both time and height.
- iii. They do not undergo chemical reactions or other removal processes.
- iv. Pollutant material reaching the ground or the top of the mixing height as the plume grows is reflected back to the plume centerline.
- 4. The equation, known as Gaussian distribution equation, is given as:





5. When concentration is required only along x-direction, i.e., in the downwind horizontal direction along the center line of the plume, then naturally, y = 0. Then eq. (2.10.1) becomes



6. When the smokes are emitted at ground level, the effective stack height (H) is zero, then the above eq. (2.10.2) gets further simplified, as



7. Values of 𝝈y and 𝝈x are not only a function of downwind distance (x) but are also a function of atmospheric stability.
Holland’s Equation:
1. The value of H used in Gaussian Distribution Equation is the effective height of the stack (chimney) and not its actual height.
2. This effective height consists of actual height (h) plus the height (𝚫h) to which the plume rises above the stack before leveling out, as shown in Fig.
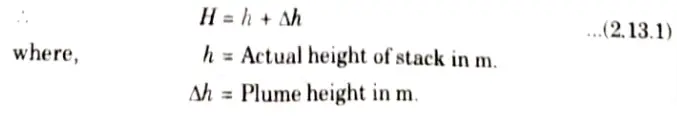
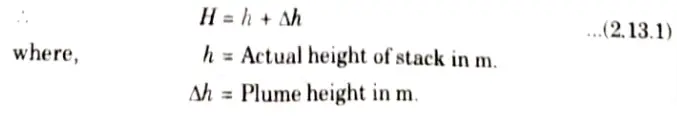
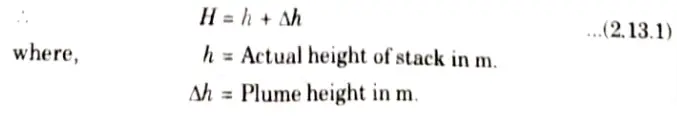
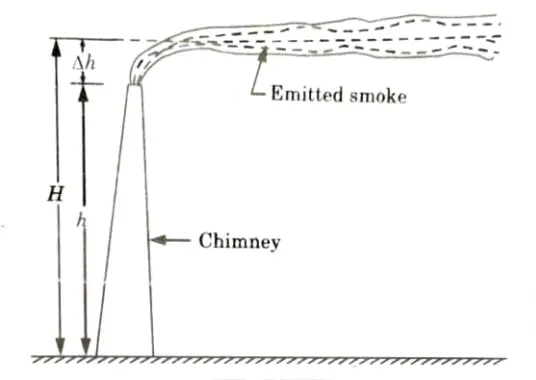


3. There exists several equations for calculating the plume height 𝚫h; out of which, Holland’s equation is often used and is given by,



4. Eq.(2.13.2) is quite suitable for computing 𝚫h from neutral conditions. For unstable conditions, the above value of 𝚫h should be increased by 10 to 20 %, and for stable conditions, it should be decreased by 20 to 10%.
5. Another frequently used equation for computing 𝚫h is given by Daviadaon and Bryant, as
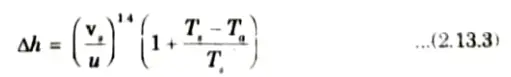
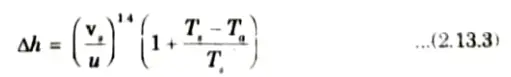
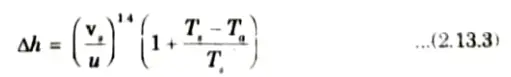
6. All the terms used in this equation have the same meaning as for eq. (2.13.2).
7. The Bureau of Indian Standards (BIS), earlier known as ISI, has through their code No. IS: 8829-1978 suggested the following empirical formulas for computing plume rise (𝚫h):
i. For hot effluents with heat release of the order of 106 cal/sec or more:



ii. For not very hot releases, and which can be counted as momentum sources above:



where, vs and D have the same meaning as in eq. (2.13.2).
d. Describe the principle of operation, advantages and limitations of the Gravitational settling chambers.
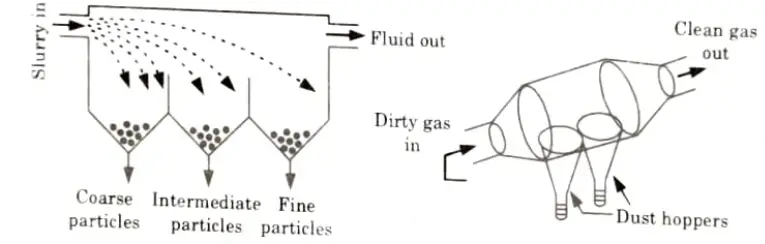
Ans. A. Gravity Settling Chamber:
- 1. It comprises of a cylindrical body with many dust hoppers as indicated in the Fig.
- 2. The unclean air enters into the compartment from one end. During this procedure, the dense particles in the air sank into the hoppers, while the clean air escaped from the opposite end.
- 3. This strategy is based on the gravity phenomena.
- 4. The emitted smokes, when made to pass through a settling chamber Fig. drop some of their larger sized particles in the chamber, under Stoke’s law.
- 5. The largest size particle (d) that can be removed with 100 % efficiency in such a chamber of length L and height H is given by,
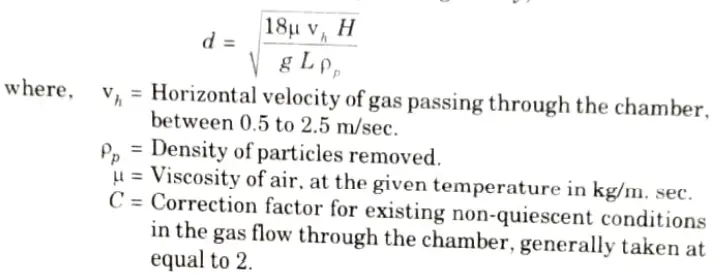
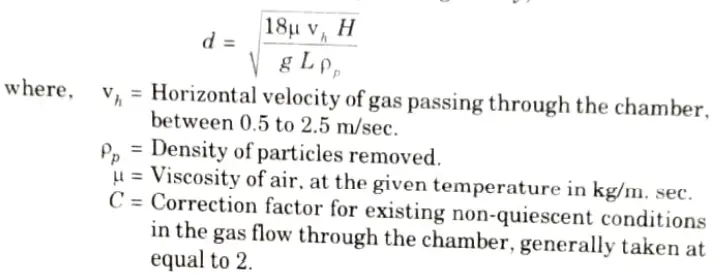
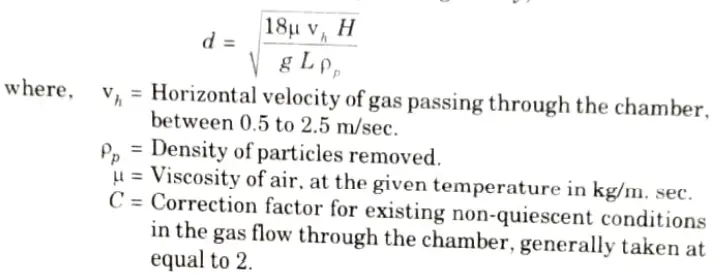


B. Advantages of Settling Chamber: Following are the advantages of settling chamber:
- 1. It has very low energy cost.
- 2. It has low capital cost.
- 3. It has low pressure drop through device.
- 4. It has excellent reliability.
- 5. It provides incidental cooling of gas stream.
C. Disadvantages of Settling Chamber: Following are the disadvantages of settling chamber:
- 1. It has large physical size.
- 2. It cannot handle sticky dust.
- 3. Its cost increases as the cost of material increases.
e. Explain point and line sources of noise pollution. Also write noise standards and sound pressure level.
Ans. Point and Line Sources:
A. Point Sources:
- 1. The SPL from an ideal point source radiator decreases at a rate of 6 dB per distance doubling.
- 2. The inverse square of the distance reduces the intensity of sound from a point source. This is referred to as the inverse square law.
- 3. The energy emitted by a point source is dispersed evenly across the surface of an expanding sphere.
- 4. The sphere’s surface area is inversely proportional to the distance (sphere’s radius) squared.
B. Line Sources:
- 1. The SPL from an infinitely long line source decreases at a rate of 3 dB per distance doubling.
- 2. This is due to the fact that the energy distribution is now over the surface of a cylinder, rather than a sphere as with the point source.
- 3. Because the expanding cylinder’s surface area is inversely proportional to distance rather than distance squared, the energy density declines simply with distance from the source rather than distance squared.
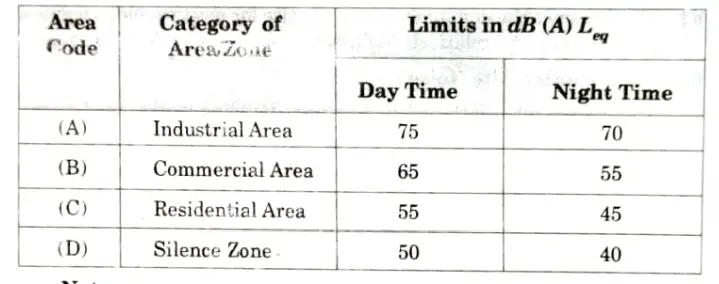
Noise Standards:


Sound Pressure Level:
- 1. The sound pressure of the faintest sound that can be heard by healthy individual is a normal about 20 micro-pascal (𝛍-Pa).
- 2. On the other hand, the loudest sound produced by a Saturn rocket at the lift off stage is about 200 Pa.
- 3. This astronomical variation in sound pressure (varying from 20 𝛍-Pa to 200 Pa) is usually avoided by expressing sound pressure on a scale based on the log of the ratio of the measured sound reference standard pressure and a pressure. Measurements on this scale are called levels.
- 4. The sound level (L) is, thus, represented as:



- 5. The unit of sound level obtained in eq. (5.6.1) is bels (B), and since it turns out to be a rather large unit, a smaller unit of decibels (dB) is generally used.
- 6. Hence, when sound level is expressed in decibels, the eq. (5.6.1) reduces to



- i. The reference standard quantity Q0 in the above equation is taken is to be equal to 20 𝛍Pa, when should pressure is measured. In that eventuality, eq. (5.6.2) reduces to sound pressure level (LP) in dB



- ii. Similarly, the reference standard quantity Q0 in eq. (5.6.2) is taken to the equal to 10-12 W/m2, when sound intensity level is measured. The sound intensity level is thus given as:
7. Sound intensity level (Li) in dB
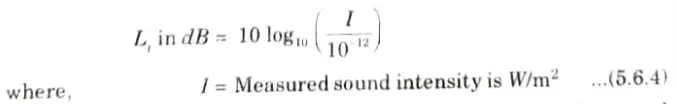
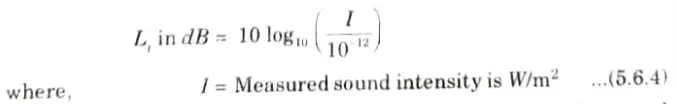
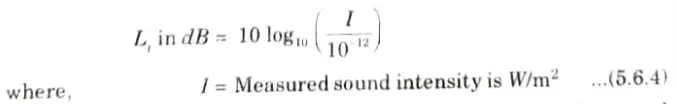
8. Out of these two terms, i.e., sound pressure and sound intensity, sound pressure level on reference scale of 20 𝛍Pa, is usually adopted to express sound levels in decibels.
Section C: Important Long Questions
a. Mention the names of some important sources generating hydrocarbons in air.
Ans. Hydrocarbons:
- 1. Hydrocarbons are compounds containing only hydrogen and carbon.
- They are generally divided into two categories:
- i. Aliphatic group of hydrocarbons which include alkanes (methanes) alkenes (olefins) and alkynes, and
- ii. Aromatic group of hydrocarbons such as benzene etc.
- 2. These are chiefly released into atmosphere by automobile exhausts.
- 3. Hydrocarbons are also released into the atmosphere by incinerator smoke, oil refinery fumes, and gasoline evaporation at service stations.
- 4. However, at the concentrations found in urban air, hydrocarbons have no negative impact on human health.
- 5. Aromatic hydrocarbons are more reactive than aliphatic ones and cause irritation of mucous membranes.
- 6. Out of three varieties of aliphatic hydrocarbons, only the alkenes, (olefins) are found to be harmful, as they are unsaturated and highly reactive in the atmosphere through photochemical reactions.
- 7. Alkanes (methenes) are simply inert hydrocarbons and do not react photochemically.
- 8. The alkynes through quite reactive, is generally not found present in the atmosphere.
- 9. Out of many aromatic hydrocarbons, Benzo (a) pyrene has been found to the most carcinogenic hydrocarbons, followed by Benzo (e) acephenanthrylene and Benzo (j) fluoranthene are less carcinogenic.
- 10. Aldehydes and ketones may also be considered under hydrocarbons, because they are found by the photochemical oxidation of hydrocarbons, as secondary pollutants in the atmosphere, although they may also be released by automobiles and incinerators along with hydrocarbons.
b. Discuss the adverse effects of carbon monoxide on human health. How can the reduction in carbon monoxide emission help mitigate these effects?
Ans. Adverse Effects:
- 1 Carbon monoxide is a colourless and odourless gas that is extremely toxic to humans.
- 2. Carbon dioxide is produced during the combustion of carbon-based fuels (CO2). But not all such combustion is complete, and this leads to the production of carbon mono-oxide.
- 3. Automobiles and industry are two of the most significant anthropogenic sources of carbon monoxide emissions.
Effects of Carbon Mono-oxide Emissions:
- Carbon monoxide poisoning is the most common cause of death in many countries around the world.
- 2. Exposures to carbon monoxide may lead to toxicity of the central nervous system and heart, severe effects on the baby of a pregnant woman, headaches and dizziness.
- 3. Problems with getting oxygen supplied to some body parts which may be life-threatening.
Reduction of CO from Atmosphere:
- 1. Install an effective ventilation system that will remove CO from work areas.
- 2. Maintain equipment and appliances (e.g., water heaters, space heaters, cooking ranges, etc.) that can produce CO in good functioning condition to encourage their safe operation and to reduce CO creation.
- 3. Consider switching from gasoline-powered equipment to equipment powered by electricity, batteries, or compressed air ifit can be used safely.
- 4. Ban the use of gasoline-powered motors or tools in inadequately ventilated spaces.
- 5. In case of possible CO exposure, give personal CO monitors audible alarms.
- 6. Test air regularly in areas where C may be present, including confined spaces.
- 7. Use a full-face piece pressure-demand self contained breathing apparatus (SCBA).
- 8. When CO levels are not excessively high, use respirators with the proper canisters in conjunction with personal CO monitoring for brief periods of time.
Section 4: Long Question for Btech 3rd Year
a. Discuss the criteria for fixing stack height.
Ans. Method to determine the minimum chimney height:
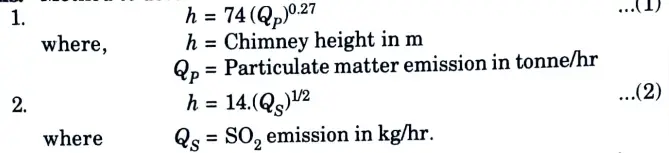
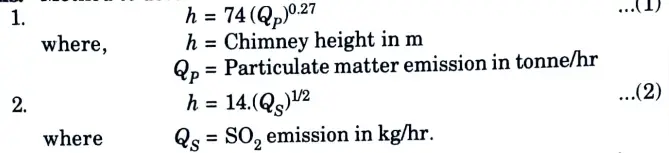
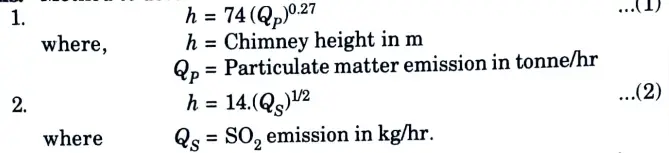
the maximum of the two heights calculated by the above equations, should be considered for adoption.
- 3. The Board has further specified that the calculated chimney height or a gives particulate matter and SO2, by the above two equations, should be further subjected to the following minimum values:
- i. For Chimneys adopted for industries in general (except thermal power plants) – 30m
- ii. For Thermal Power Plants:
- a. Above 200 MW and below 500 MW – 229m
- b. Above 500 MW capacity – 275 m
- 4. As per these standards, the minimum stack was to be 30 m, but later on, the Board, has relaxed these provisions for chimneys of Boilers and Diesel Generator Sets to lower minimum values, as per their latest guidelines contained in “Emission Regulations Part IV.
- 5. According to these provisions, the minimum stack height for a boiler, generating steam@less than 2 t/hr will be 9 m; and that for a boiler producing steam@ 30 t/hr, it will be 30 m.
- 6. Intermediate minimum values for intermediate capacities are specified between 9 to 30 m.
- 7. Similarly, for diesel generator sets of different capacities (KVA); the minimum stack height to be kept is only 1.5 to 3.5 m more than the height of the building, and is to expressed as:



b. What is a high volume sampler? Explain its salient features and procedure adopted for the sampling and measurement of suspended particulate matter.
Ans. A. High Volume Samplers: High volume samplers are instruments used to collect samples of air particles to monitor ambient air quality. They are in widespread use all over the world to measure air pollution. They work on Stokes law for collection of particulates.
B. Salient Features of High Volume Air Sampler:
- 1. Heavy Duty Blower,
- 2. Orifice flow meter : measures flow,
- 3. Time Totalizer: records time,
- 4. Programmable Timer: measures time,
- 5. Instrument Cabinet: acts as protection,
- 6. Filter Holder assembly: holds the filter in position,
- 7. Voltage Stabilizer:guards against voltage fluctuation,
- 8. Detachable roof : allows passage of air and protects filter.
C. Procedure for the Sampling and Measurement of Suspended Particulate Matter:
- 1. In these samplers, air-borne suspended particulates (SPM) are measured by passing air at a high flow-rate of 1.1 to 1.7 cubic metres per minute through a high efficiency filter paper which retains the particles.
- 2. The equipment measures the volume of air sampled, while the number of particles collected is estimated by measuring the change in weight of the filter paper as a consequence of the sampling.
- 3. The passage for air reaching the filter is designed to prevent heavier settleable dust particles from reaching the filter (by provision of cyclone) thus measuring the concentration of Suspended Particulate Matter (SPM) in atmospheric air.
Section 5 Important Long Questions
a. How is a low voltage two stage electrostatic precipitator different from a high voltage single stage electrostatic precipitator ? Show the schematic view of a plate type electrostatic precipitator and mention the size efficiency relationship.
Ans. A. Difference: The single stage electrostatic system is designed to charge and collect particulates using the same electric field. The charging and collecting fields of a two-stage electrostatic precipitator are independent of one another.
B. Size-Efficiency (Particle Size-Collection Efficiency) Relationship of an Electrostatic Precipitator:
- 1. The designer must estimate the particle-migration velocity before determining the collection area and efficiency.
- 2. The rate at which a charged particle migrates towards the grounded collection electrode.
- 3. Variables affecting particle velocity are particle size, the strength of the electric field, and the viscosity of the gas.
- 4. How readily the charged particles move to the collection electrode is denoted by the symbol, w, called the particle-migration velocity.
- 5. The migration velocity is expressed in following equation:



- 6. As shown in above equation, migration velocity depends on the voltage strength of both the charging and collection fields.
- 7. The migration velocity also depends on particle size; larger particles are collected more easily than smaller ones
C. Schematic view of a Plate type Electrostatic Precipitator:
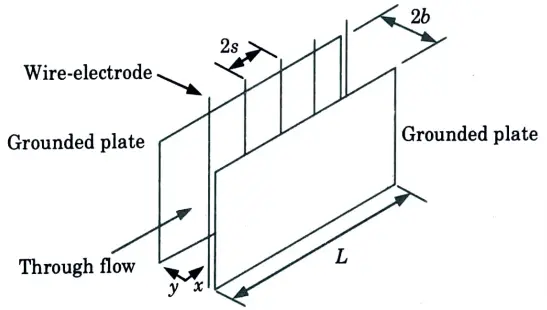


b. Derive the relation between temperature and altitude and explain its relation with atmospheric stability.
Ans. A. Temperature-Altitude Relationship:
1. Let us imagine an air parcel issuing from a stack. As it begins its upward journey, its temperature, pressure and volume will be changing. According to the first law of thermodynamics, we can write



W = Work done on the system which is equal to P.dV.
2. Substituting these terms in eq. (1), we get



4. Differentiating eq. (3), we get
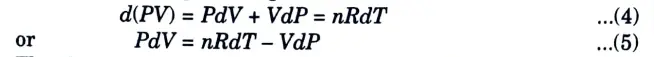
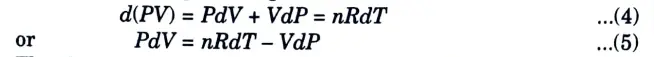
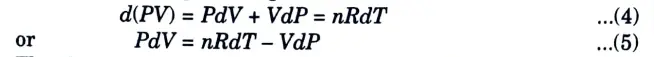
5. The thermodynamics relationship between the heat capacity at constant volume (Cv) and constant pressure (Cp) is given by,
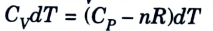
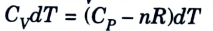
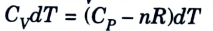
6. Substituting these in eq. (1), we get
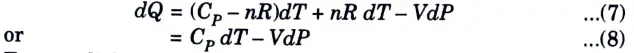
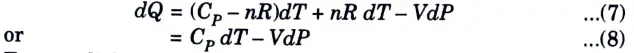
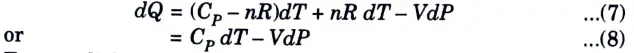
7. For an adiabatic system there is no heat transfer from the system to the surroundings. Hence, dQ is zero. Therefore, it follows that



Eq. (9) describes how atmospheric temperature changes with pressure.
8. Expressing the rate of change in temperature with altitude as a product and substituting in eq. (7), we get



9. The product V.𝛒 is equal to 1 and the expression simplifies to,



The negative sign indicates that the temperature decreases with altitude.
10. Substituting the value ofg (9.806 m/sec2) and Cp (1005 J/kg °C) in eq. (11) yields.



The differential dY dz is called the adiabatic lapse rate and denoted by A which corresponds to = 10 °C per 1000 metres or 5.4 °F/km.
B. Relation between Temperature and Altitude and Atmospheric Stability:
- 1. Atmospheric stability depends upon the temperature changes undergone by an air mass as it rises or sinks, which in turn relate to pressure changes.
2. Relationships between pressure and temperature lead to a simple linear relation between temperature and altitude for rising sinking air. - 3. This is known as the Dry Adiabatic Lapse Rate (DALR), and is equal to 9.8 °K/km.
- 4. Air lifted up will cool at this rate due to reduction in pressure; air sinking will warm at this rate due to pressure increases.
- 5. Thus, rising air is said to cool or warm adiabatically when its temperature changes are due entirely to pressure changes.
Section 6 Long Question choose any one
a. Discuss the pollution control process of gaseous contaminants through absorption.
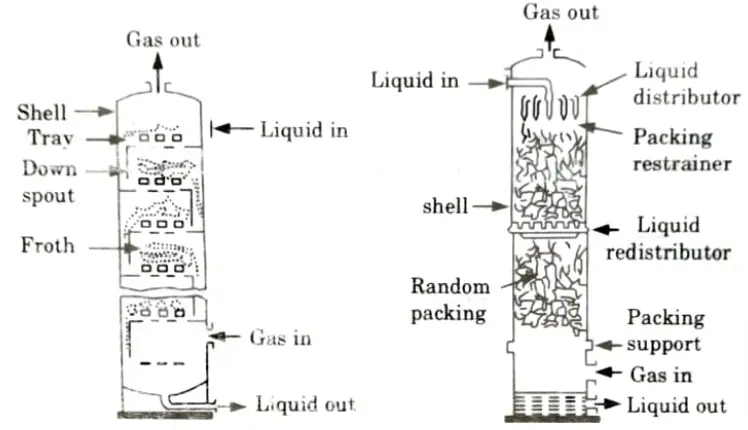
Ans. Absorption Units: Absorption units includes spray towers, plate towers, packed towers, and venturi scrubbers.
- 1. Spray towers and venturi-scrubbers can be used to remove particulate pollutants from the air at the same time.
- 2. Scrubbers are often less successful in eliminating gaseous pollutants than towers, but then, towers get regularly blocked by particle matter.
- 3. Other effective devices for removing gaseous pollutants, ie. plate towers and packed towers, are shown in Fig.1 and Fig.2, respectively.
- 4. These absorption units work on the principle of transfer of the pollutants from the gas phase to the liquid phase.
- 5. In other words, the pollutants from the dirty gas get absorbed in the liquid, through which the gas is made to pass, in these units
- 6. Diffusion and dissolution are both methods of absorption.
- 7. The efficiency of these devices, naturally, depends upon the solvent (liquid), through which the gas is made to travel.
- 8. When water is used as the solute, the removal is restricted only to a few inorganic gases, such as NH3, Cl2, and SO2.
- 9. All such absorbent units must be properly designed for the given conditions, before one of them is adopted.
- 10. Care should also be taken to ensure that the pollutants from air to water, may not cause heavy and uncontrollable water pollution.


b. With the help of suitable diagram explain the working principle of spray tower.
Ans. A. Spray Tower:
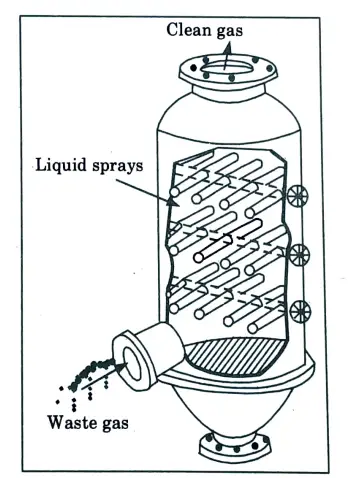


- 1. Spray tower is new gas-cleaning equipment that is widely used in industrial waste gas purification and treatment.
- 2. It consists of tower body, liquid storing tank, air inlet, spray level, packing layer, demister, air outlet, manhole, etc.
- 3. If the pollutants are highly soluble or a chemical reagent is added to the liquid, a spray tower can be very effective in removing them.
- 4. It is ideal for exhaust gas purification and treatment of chemistry, electrical, metallurgy, hardware manufacturers, casting factories, etc.
- 5. We can produce it according to the information provided by customers, such as capacity of waste gas treatment, concentration, model, etc. Besides, we can manufacture it according to the drawings provided by customer.
B. Working Principle of Spray Tower:
- 1. Spray tower achieves gas purifying by neutralizing the acid-base properties of exhaust gas.
- 2. Contacting exhaust gas with liquid, pollutants of exhaust gas will be transferred into liquid, then the purified gas is separated from polluted liquid and discharged into the atmosphere.
- 3. When exhaust gas enters into spray column through inlet and passes through packing plate, acidic and alkaline components in exhaust gas will be fully absorbed and neutralized by sodium hydroxide solution.
- 4. After purifying, exhaust gas will be dehydrated through demister, and discharged into the atmosphere.
- 5. The absorption liquid at the bottom of spray tower is pressurized by the pump and sprinkled from tower top.
- 6. Finally, the liquid returned to the tower bottom for recycling. The purified exhaust gas can reach national discharging standards.
Section 7 Long Question
a. Explain the concept of equivalent continuous energy level (Leq).
Ans. Equivalent Continuous Energy Level (Leq) Concept:
- 1. Leq is the statistical value of sound pressure level that can be equated to any fluctuating noise level.
- 2. This value is called the equivalent continuous equal energy level or equivalent noise level (Leq).
- 3. Thus, Leq is defined as the constant noise level, which, over a given time, expands the same amount of energy, as is expanded by the fluctuating levels over the same time.
- 4. It is expressed as:
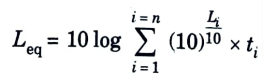
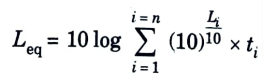
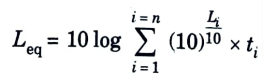



- 5. The equivalent noise level ( Leq) constitutes an important parameter for evaluating the impact of fluctuating noises of all kinds, such as from aircraft, street and road traffic, rail traffic, industrial machines, sports stadiums, play grounds, etc. 6. Moreover, the duration in hours, over which Leq is worked out for a given site, is further mentioned in bracket, such as Leq(8) which means that Leq is based on 8-hour measurement; when, however, no such time is mentioned, then Leq always corresponds to one hour measurement.
- 6. Moreover, the duration in hours, over which Leq is worked out for a given site, is further mentioned in bracket, such as Leq(8) which means that Leq is based on 8-hour measurement; when, however, no such time is mentioned, then Leq always corresponds to one hour measurement.
b. Explain in detail the outdoor noise propagation and indoor noise propagation in relation with noise pollution and control.
Ans. A. Outdoors Noise Propagation:
- 1. From the source, sound waves travel in a continuously extending spherical wave-front.
- 2. When a point source emits a specific amount of sound energy, that energy is concentrated in a single point at the source.
- 3. At a distance from the source, the same energy is distributed over a sphere.
- 4. The greater the distance from the source, larger the surface over which the energy is dispersed.
- 5. The sound energy is dispersed over an imaginary sphere with a surface that grows in proportion to the square of the distance from a point Source.
- 6. The surface of the sphere grows 4 times with each doubling of the distance from the source.
- 7. The sound hence rapidly declines with the distance from the source.
- 8. Each doubling of the dist ance from the point source yields a 6 dB reduction of the sound level.
B. Indoor Noise Propagation:
- 1. The sound wave hits building construction surfaces before it is significantly attenuated.
- 2. The sound field inside is not spherical and is determined by the shape and acoustical qualities of the surfaces.
- 3. The volume of the room and the distances between the sound source, the building construction surfaces and the listening point are also important
- 4.. The sound in a certain listening point in a room is composed of the direct sound and the reflected sound.
- 5. The direct sound is the sound that has not yet been reflected in a surface.
- 6. The sum of all reflected sound is called the reverberant sound field.
- 7. It consists of all sound that has been reflected once, twice or more in the building construction surfaces.
- 8. The sound reflected one time is called 1st reflections, two times 2nd reflections etc.
- 9 If the surfaces were perfectly absorptive, there would be no reflections at all.
- 10. In reality, there is always a loss of energy when a sound wave hits a wall.
- 11. The air also absorbs some of the sound wave’s energy
- 12. The sound absorption is frequency dependent. High frequency sound is frequently easier to absorb than low frequency sound.
6 thoughts on “Air and Noise Pollution Control Question paper with answer, Quantum Notes”